Founded in Shimizu, Japan in 1976, Intelligent Actuator Incorporated (IAI) has over forty years of experience in the design and manufacture of customized medical and industrial robotics.
Built on “Quality and Innovation,” IAI is a leader in the research and development of advanced energy-efficient and affordable Cartesian, SCARA, and Tabletop robots. From entry-level to high-speed models, IAI provides complete programmable systems and components to fulfill any and all of your medical testing and industrial production needs.

IAI systems and components cover a full range of medical applications including infectious disease testing, molecular diagnostics, tissue and blood sampling, genomic research, cancer research, pharmaceuticals, and medical device production. The latest system project of IAI integrated a 3-axis Cartesian Robot into a cancer screening apparatus to assist in a ground-breaking process to detect and identify several types of cancers.
Currently, IAI is assisting the medical community by providing robotic components for Covid-19 molecular lab testing systems. The current pandemic has created an urgent need for multiple testing and diagnostic tools to be readily available for use worldwide. The pressing concern is for expedited delivery, installation, and programming to quickly integrate and optimize production.
IAI expedites delivery by stocking hundreds of electric actuator components that are easily combinable to meet precise customer specifications. This production method reduces turn-around time to a minimum and provides complete pre-assembled products ready for use upon delivery. Customizable options based on specified applications include: Size/footprint, speed/acceleration, payload, XYZ-ZR-axis configurations, and orientation of actuators.
IAI Cartesian robots come in hundreds of configurations in 2-axis to 6-axis combinations and are equipped with a High-Resolution Battery-less Absolute Encoder as standard. (No battery, No maintenance, No replacement, and No home return since the current position is always known). There is on-board programming for ease of operation. A ZR-Axis (vertical/rotation) option is now available to enable camera systems for image processing and analysis while load moving.
Straight forward programming language and pre-formatted products make it easy to integrate all IAI products with other components and to be up and running quickly.
There are four distinct IAI Robot Series to meet your needs:
- ICSB Series – Full Cartesian gantry systems (up to 3-meter spans) for large payloads and robust applications. Offering 954 variations. 726 of which are compatible with the battery-less absolute encoder.
- The IK ROBO Cylinder ® Series – An affordable compact option for lighter weight multi-axis applications. Offering 516 user options. The battery-less absolute encoder is standard.
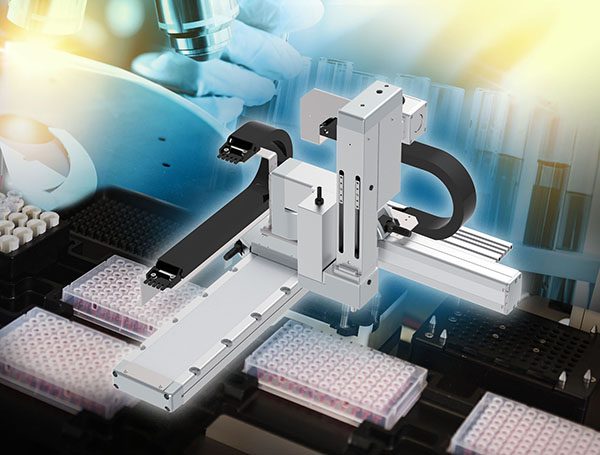
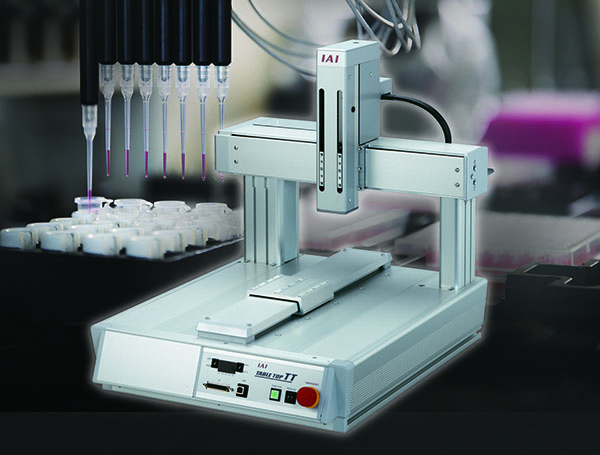
- TTA Table Top system – a compact design that is extremely flexible, precise, and multi-functional. No assembly required. Arrives pre-fabricated with a built-in controller ready for use. Excellent for medical office applications. Offering eight types for various operations as in pick and place and sorting applications. The dedicated ZR-axis option enables video image processing and analysis for micro-dosing applications in biotechnology.
- IXP/IXA SCARA Series – The IXP model is ultra-compact and cost-effective using 24VDC stepper motors to provide an affordable entry-level into cleanroom automated testing. The IXA model uses 200VAC servo motors and has high-speed payload capabilities. Both models in the series have excellent position repeatability and are equipped with battery-less absolute encoders.

Connectivity between each series is also possible to provide the ability to create a complete and seamless production line system. A full package of actuators, motors, encoders, controllers, software, brackets, cables, and cable management are included with each package along with on-site training to finalize the operation of the project. A comprehensive support system of trained technicians stand by to answer all of your questions before, during, and after installation. Please contact the office nearest you and click the link below for more information:
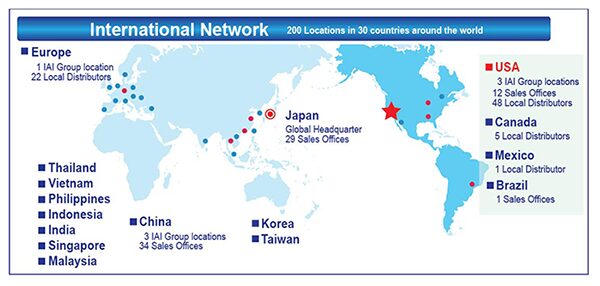
USA Headquarters & Western Region
2690 West 237 Street, Torrance, CA 90505
(310) 891-6015
Midwest Branch Office
110 East State Parkway, Schaumburg, IL 60173
(847) 908-1400
Southern Branch Office
1220 Kennestone Circle, Suite 108, Marietta, GA 30066
(678) 354-9470
Japan Headquarters
577-1 Obane, Shimizu-ward, Shizuoka-city, Shizuoka-pref.


