Canary Medical is a medical data company focused on the development and commercialization of its patented implantable sensor technology.
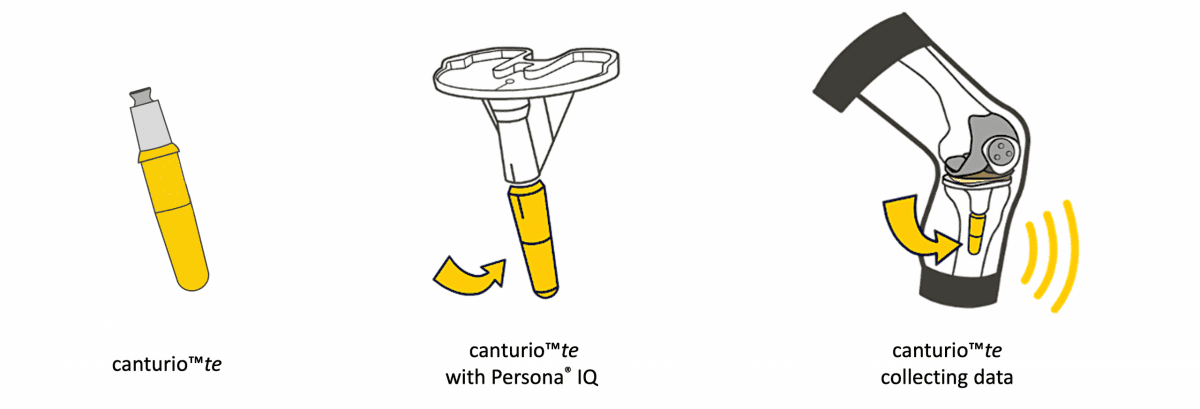
Canary Medical today announced that it will present data in a podium presentation assessing Persona IQ® an investigational smart knee implant that combines Zimmer Biomet’s Persona® The Personalized Knee® with Canary Medical’s proprietary implantable canturioTM te tibial extension sensor technology, at the American Academy of Orthopedic Surgeons (AAOS) Annual Meeting being held from August 31st to September 3rd in San Diego, CA. The canturioTM te tibial extension is currently under FDA review and is not available for commercial use or sale.
Conference Details:
Conference: American Academy of Orthopaedic Surgeons (AAOS)
Paper Title: The Talking Knee Is a Reality: Remote Patient Monitoring Prosthesis for Total Knee Arthroplasty
Presenter: Fred D. Cushner, MD, FAAOS, Hospital for Special Surgery
Session: Adult Reconstruction Knee IV
Paper Number: 459
Date and Time: 9/2/2021, 9:00 AM (PT), Ballroom 6A
The presentation abstract will be available on the AAOS website on August 31st here. Canary Medical will also be showcasing its technologies at its booth #1025 in Hall B1 as well as in Zimmer Biomet’s booth #2535 in Hall C.
Canary Medical was conceived and created by a team of medical device developers with active implantable experience, clinician guidance, and IoT experts with the vision that (1) healthcare transformation requires better and cheaper healthcare data, (2) better monitoring and better data will produce better outcomes at lower costs, and (3) patients own their healthcare data and should be compensated for its use. Canary Medical’s patented implant and data management ecosystem technology provides the vehicle to implement its vision. Canary Medical is led by a team of experienced entrepreneurs, researchers and data scientists globally regarded for their expertise in medical device design, development and data informatics.
