What To Know
- “With the FDA clearance and the reach of the global medical device sales team, every cath, EP, and IR lab in the country that has a Siemens Artis zee will now have the opportunity to reduce their radiation dose by 85%1 without compromising image quality,”.
- “We recently completed a clinical trial designed to evaluate how this novel technology might reduce radiation exposure in the cath lab.
March 4, 2021
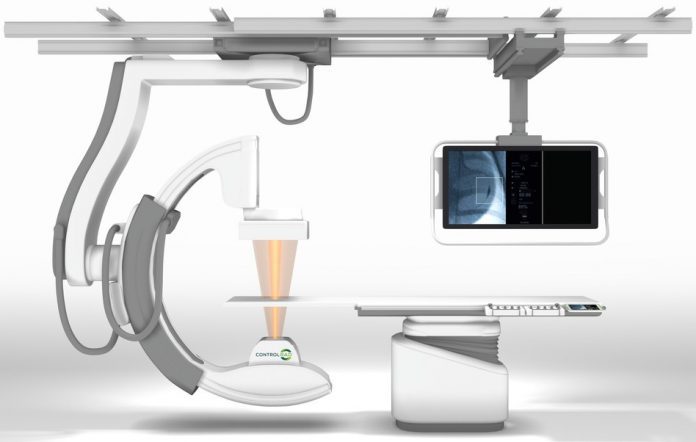
ControlRad Select technology utilizes proprietary semi-transparent filters, a user-interface tablet, and image processing algorithms, which are retrofitted onto customers’ existing Siemens Artis zee interventional imaging systems.
In preparation for the commercialization efforts, ControlRad has entered into an exclusive agreement with Boston Scientific Corporation to sell the ControlRad Select technology.
“With the FDA clearance and the reach of the global medical device sales team, every cath, EP, and IR lab in the country that has a Siemens Artis zee will now have the opportunity to reduce their radiation dose by 85%1 without compromising image quality,” stated Guillaume Bailliard, CEO of ControlRad.
“The health risks to the medical staff due to lifetime radiation exposure in cath labs have been well documented, including increased incidence of cataracts, atherosclerosis and even left-brain tumors,” stated Simon Dixon, MD, Chief of Cardiology at Beaumont Hospital, Royal Oak, Michigan. “We recently completed a clinical trial designed to evaluate how this novel technology might reduce radiation exposure in the cath lab. I have long been passionate about finding innovative ways to improve safety for my colleagues in the lab while they are performing lifesaving procedures for our patients.”
Additional details can be found here.