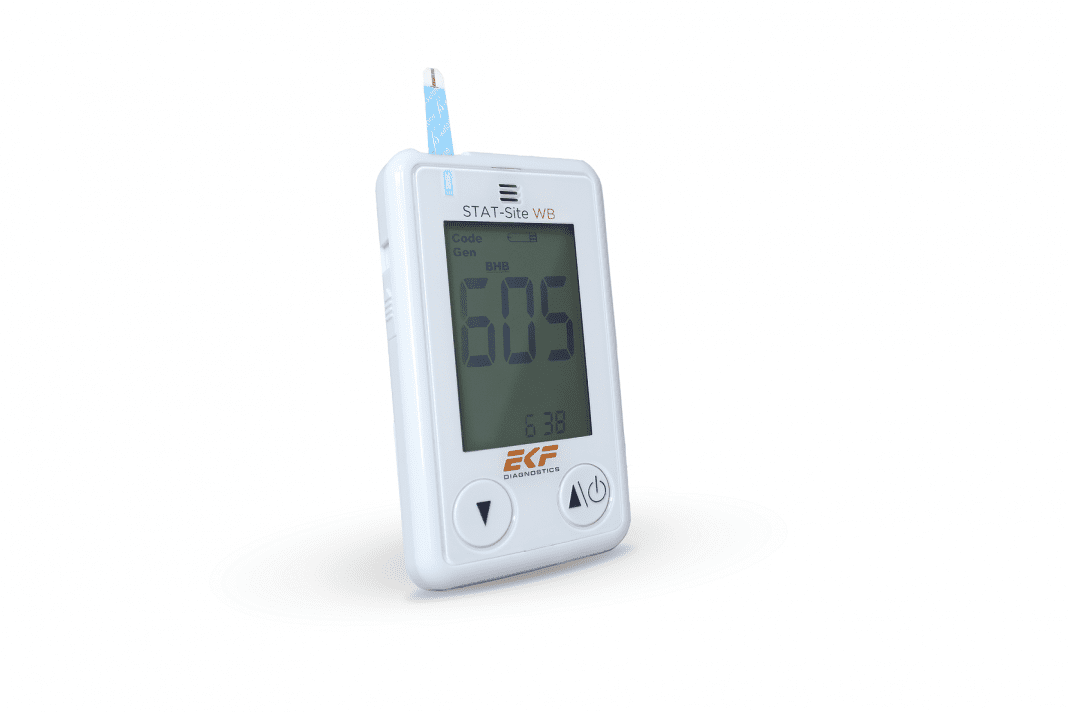
EKF Diagnostics, the global in vitro diagnostics company, announces that it will be demonstrating its new STAT-Site® WB dual analyte measurement system at the 2021 AACC Annual Scientific Meeting & Clinical Lab Expo.
As EKF’s first face-to-face event since COVID-19 restrictions began, this will be the premiere of the new handheld analyzer for reliable and efficient β-ketone and glucose measurement from whole blood in seconds. Visitors will be able to see firsthand how easy it is to use the CLIA waived and CE marked device in professional settings to monitor the effectiveness of diabetes control programs. EKF will also be introducing its newest hemoglobin analyzer for the US market, the Hemo Control, which provides near patient hemoglobin and hematocrit results in one test as quickly as 25 seconds.
EKF’s team of point-of-care and central laboratory diagnostics experts at the Georgia World Congress Center in Atlanta, U.S., September 26-30, will also highlight the Lucica® Glycated Albumin-L. Manufactured by Asahi Kasei Pharma, it is a specific test for glycated albumin (“GA”) and is FDA cleared for sale in the U.S. where it is sold exclusively by EKF Diagnostics. This established test enables intermediate term glycemic monitoring of diabetics over the preceding 2-3 weeks.
The Lucica® Glycated Albumin-L is a quantitative, enzymatic test for use on compatible clinical chemistry analyzers as a “user defined” method. The Lucica® method has appeared in numerous published studies.
Glycated Albumin levels are not affected by the life span of red blood cells (“RBC”), and therefore it is a useful substitute when the HbA1c interpretation is problematic such as in the presence of hemoglobinopathies, iron deficiency, and anemias. Another key feature of glycated albumin is that it changes more rapidly and markedly than HbA1c. Therefore, GA is a good marker to monitor the effects of changes in therapy in patients with diabetes when more rapid treatment responses are needed.
The STAT-Site® WB and Lucica® Glycated Albumin-L test are part of a suite of other diabetes care products that EKF will be showcasing at 2021 AACC. These include its Beta-Hydroxybutyrate LiquiColor® Assay (B-HB) and Nitro-Tab Ketone Tablets, both of which provide quick and simple tests for ketosis.
Other product highlights on EKF’s stand for point-of-care and central laboratory testing include its hemoglobin analyzers, the hand-held DiaSpect Tm and the HemoPoint® H2 which offers both hemoglobin and hematocrit results in one test within 30-60 seconds. Additional analyzers on show include the Biosen RUO glucose and lactate analyzer.
EKF will also discuss its growing EKF Life Sciences business which provides contract manufacturing services, as well as manufacturing diagnostic enzymes for use in medical diagnostics, pharmaceuticals and industry. This follows recent significant investment in its longstanding fermentation facility in Elkhart, Indiana, where it has been manufacturing enzymes and related biomolecules from bacterial fermentation since 1983.