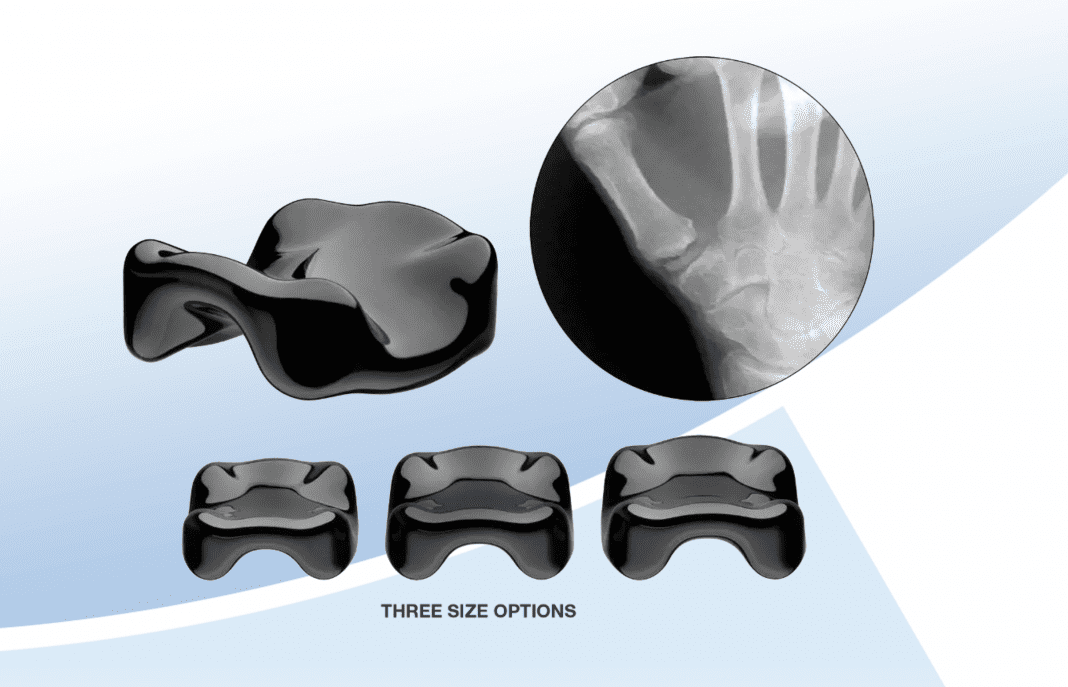
Ensemble Orthopedics Inc, a privately held company, today announced the 35th implantation of its interpositional CMC device for the treatment of carpometacarpal (CMC or thumb) osteoarthritis.
The company will showcase the Ensemble CMC™ at booth #526 during the 76th Annual Meeting of the American Society for Surgery of the Hand (ASSH) on September 30-October 2 in San Francisco.
The surgery was performed on September 1, 2021 at Ocala Regional Medical Center in Ocala FL by Dr. Nirav Gupta who stated, “The beauty of the Ensemble CMC™ is its simplicity. By leaving surrounding soft tissue and bony structures intact, the procedure allows for decreased operative time. Most importantly, the patients I have implanted with this device demonstrate reduced pain levels and earlier return to function than other surgical treatment options I have used. This implant allows me to expand my practice to patients with moderate, isolated CMC osteoarthritis of the thumb in which symptoms persist despite non-operative management.”
The FDA-cleared Ensemble CMC™ is the first implant in a full product line of minimally invasive, interpositional joint replacement devices for the hand and wrist. “We are encouraged by the short-term clinical outcomes and the pattern of repeated use amongst our trained surgeons,” said William Ogilvie, Co-founder, President and CEO of Ensemble Orthopedics. “These results support the concept of using minimally invasive techniques for hand arthroplasty that provide patients a quicker return to normal function.”
The Ensemble CMC™ was designed to expand the treatment options for osteoarthritis of the carpometacarpal joint in a broader range of patients. The minimally invasive, simple surgical procedure inserts an implant through a small incision without disrupting the surrounding soft tissue structures that stabilize the joint. The device is manufactured from pyrocarbon, a strong, low-friction, biocompatible material which has been shown to provide an ideal surface for sliding contact with bone and cartilage.
Ensemble Orthopedics, a medical device company based in Austin TX, develops proprietary, interpositional implants designed to employ minimally invasive surgical techniques, thereby retaining stabilizing tendons and ligaments, for the treatment of arthritis in the hand and wrist.