What To Know
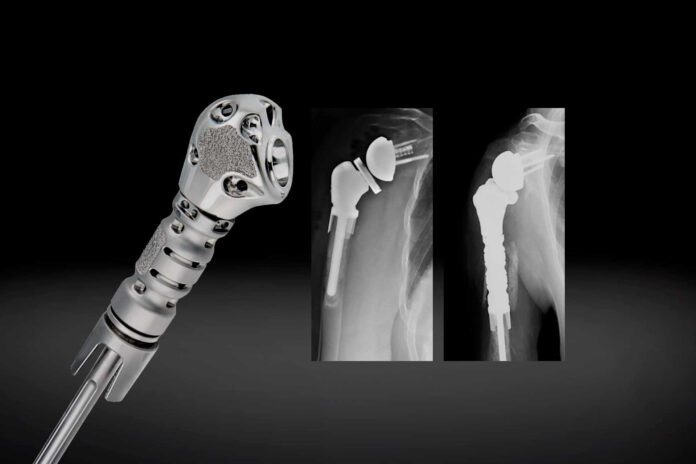
- “This modular prosthesis is capable of reconstructing a patient's humerus with as little as 50mm of bone loss, while also being able to address the most challenging cases, including patients who have a resection below the deltoid insertion, through the use of additional modular middle segments.
- “The Equinoxe Humeral Reconstruction Prosthesis is designed to treat patients with a wide range of proximal humeral bone loss,” said Pietro Ruggieri, MD, PhD (Department of Orthopedics and Orthopedic Oncology, University of Padova, Italy), a design surgeon.
Exactech, a developer and producer of innovative implants, instrumentation and smart technologies for joint replacement surgery, announced today that its unique Equinoxe® Humeral Reconstruction Prosthesis is now available for clinical use in Europe.
The Humeral Reconstruction Prosthesis – the first modular device available in the U.S. for significant proximal humeral bone loss and reconstruction after tumor resections – is designed for intraoperative flexibility and patient-specific sizing. Addressing both straightforward and challenging cases, the prosthesis offers numerous options to achieve stability regardless of proximal humeral resection length. It is used in primary or revision arthroplasty procedures as well as anatomic oncology procedures.
Exactech notes first introduced in the U.S. in 2015, the humeral reconstruction prosthesis has been successfully used to treat more than 1,500 patients. It is now available in Italy, France, Spain, Germany and Great Britain.
“The Equinoxe Humeral Reconstruction Prosthesis is designed to treat patients with a wide range of proximal humeral bone loss,” said Pietro Ruggieri, MD, PhD (Department of Orthopedics and Orthopedic Oncology, University of Padova, Italy), a design surgeon. “This modular prosthesis is capable of reconstructing a patient’s humerus with as little as 50mm of bone loss, while also being able to address the most challenging cases, including patients who have a resection below the deltoid insertion, through the use of additional modular middle segments. I am thrilled that this remarkable device is now available for treatment of patients in Europe.”
A key feature of the Humeral Reconstruction Prosthesis is its modularity, with a platform stem and multiple modular segments permitting reconstruction between 50mm – 212.5mm and 60mm – 222.5mm, and several soft tissue fixation options. The system is designed to provide biomechanical advantages including rotational stability, due to its unique plasma/hydroxyapatite (HA) coated collars intended for fixation and optimal fit.
“At Exactech we challenge our development teams to create products that solve unmet clinical needs, and our Humeral Reconstruction Prosthesis is an innovative solution that was designed to address the most difficult humeral challenges shoulder surgeons face,” said Chris Roche, Sr. Vice President, Extremities. “The Humeral Reconstruction Prosthesis was the first reverse TSA device cleared for use in the U.S. for significant resection of the proximal humerus.”
“It incorporates many unique design features intended to mitigate complications associated with these challenging cases,” Roche added, “including collars available in 17 different sizes to achieve rotational stability and fixation for the full range of bone sizes. It also provides numerous proximal body options to achieve joint stability while also lateralizing the deltoid. Our surgeon and engineering teams are proud to deliver this unique and innovative shoulder solution to European market.”
Exactech advises along with Dr. Ruggieri, the Humeral Reconstruction Prosthesis design team includes other experienced revision upper extremity specialists and orthopaedic oncology surgeons: Pierre-Henri Flurin, MD, Clinique du Sport (Merignac, France); Parker Gibbs, MD, University of Florida (Gainesville, Fla.); Mark Scarborough, MD, University of Florida (Gainesville, Fla.); Thomas Wright, MD, University of Florida (Gainesville, Fla.); and Joseph Zuckerman, MD, NYU Langone Orthopedic Hospital (New York, N.Y.). More here.