What To Know
- “We have seen increasing early use of our NeuRx device in spinal cord injured patients and, coupled with the use of our TransAeris system over this past year intended to help ICU patients get off mechanical ventilation earlier under the Emergency Use Authorization program, we hope the mounting evidence and this designation along with our ongoing clinical studies will help move the TransAeris device along the path for quicker approval.
- TransAeris builds on the success of another Synapse Biomedical technology, the NeuRx® Diaphragm Pacing Stimulation (NeuRx DPS) System, which has been FDA and CE Mark approved since 2008 for people with Spinal Cord Injury (SCI) and has successfully reduced or eliminated the need for mechanical ventilation.
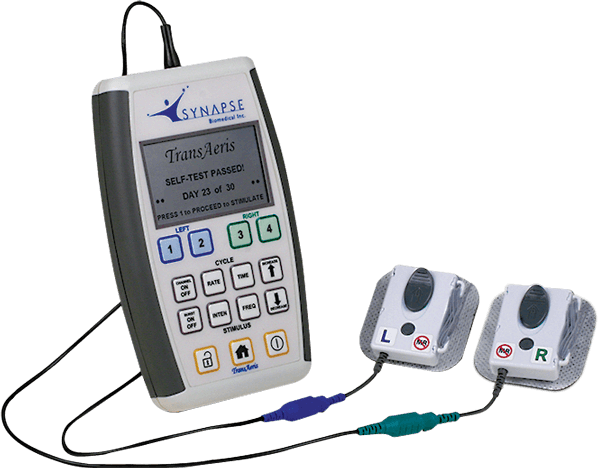
Synapse Biomedical, Inc®. announced today that the FDA has granted Breakthrough Therapy Device designation to TransAeris®, a temporary percutaneous intramuscular diaphragm stimulator designed to aid in weaning from mechanical ventilation.
TransAeris system is a temporary percutaneous intramuscular diaphragm stimulator intended for patients identified with a risk of prolonged mechanical ventilation: undergoing high-risk open cardiac surgery, lung transplant, thoracoabdominal aortic aneurysm surgical procedures or in patients that have already failed to wean and have been on mechanical ventilation for 96 or more hours, to prevent or treat ventilator induced diaphragm dysfunction (VIDD). Diaphragm Pacing may enhance recovery after surgery in patients at risk or on prolonged mechanical ventilation.
The FDA’s Breakthrough Therapy Device Program creates a path for innovators to get their medical devices to market faster. The program targets novel devices that have the potential to provide patients with a more effective treatment or diagnosis for life-threatening or irreversibly debilitating diseases and conditions. This program provides patients and health care providers with timely access to these medical devices by expediting medical devices development, assessment, and review, while preserving the statutory standards consistent with the FDA’s mission to protect and promote public health.
“The Breakthrough Device designation for our TransAeris temporary diaphragm stimulation system is built upon our 20-year experience with NeuRx diaphragm pacing,” said Anthony Ignagni, President & CEO. “We have seen increasing early use of our NeuRx device in spinal cord injured patients and, coupled with the use of our TransAeris system over this past year intended to help ICU patients get off mechanical ventilation earlier under the Emergency Use Authorization program, we hope the mounting evidence and this designation along with our ongoing clinical studies will help move the TransAeris device along the path for quicker approval.”
TransAeris builds on the success of another Synapse Biomedical technology, the NeuRx® Diaphragm Pacing Stimulation (NeuRx DPS) System, which has been FDA and CE Mark approved since 2008 for people with Spinal Cord Injury (SCI) and has successfully reduced or eliminated the need for mechanical ventilation. TransAeris was created to assist patients with prolonged mechanical ventilation on a temporary basis up to 30 days, simplifying the external features of the NeuRx DPS system and distilling them into a single patient, 30-day use, disposable device. Since receiving CE mark in Europe for TransAeris, several centers in Europe and the Middle East, including BGU Murnau (Germany), have also used TransAeris successfully in acute spinal cord injury and polytrauma patients.