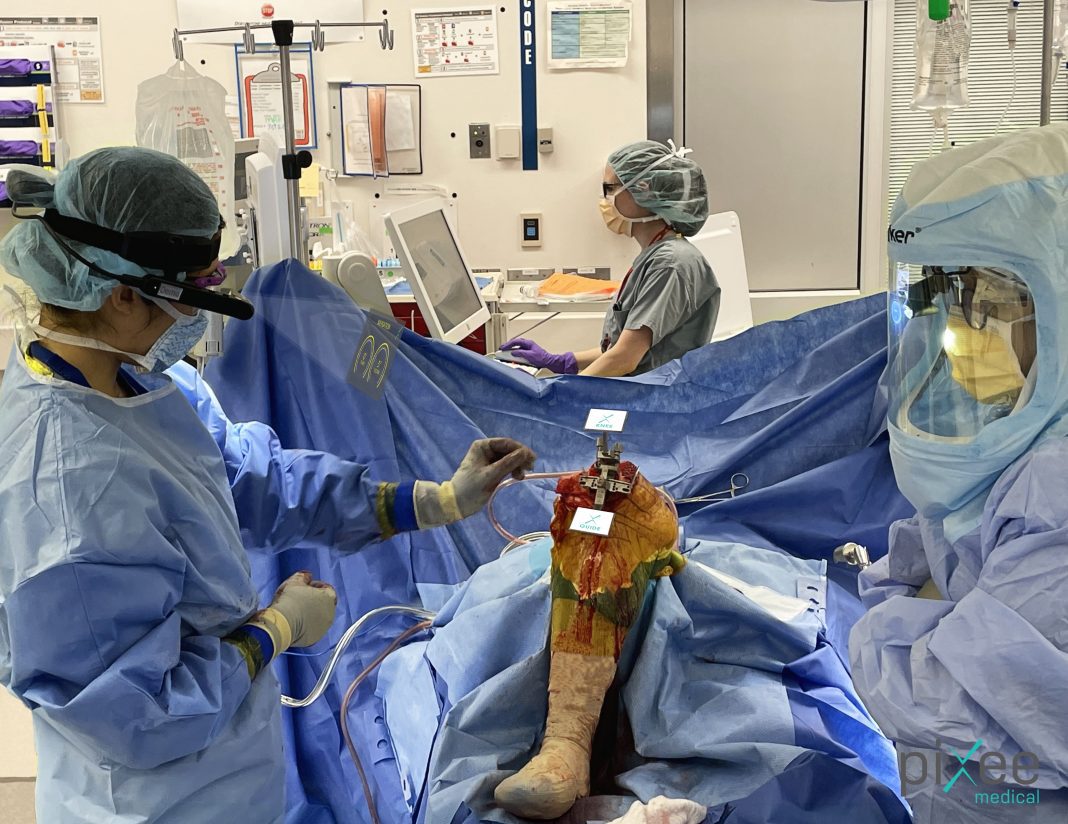
Pixee Medical, a pioneer in digitally augmented surgery technology, announces that Dr Antonia Chen performed the first Knee+ surgeries in the USA at the Brigham and Women’s Hospital in Boston (MA).
Knee+ is a patented platform designed to help orthopedic surgeons perform Total Knee Replacement surgeries by providing real-time 3D positioning of instruments, right in their field of view. Knee+ is intuitive and requires minimal training since it does not change the overall workflow for surgeons. Knee+ consists in a proprietary software using unique computer vision algorithms and running on augmented reality smart glasses, without bulky capital equipment or disposables required.
After the first Knee+ surgery in June 2020 in Lariboisière Hospital (Paris, France), Pixee Medical began commercialization in Europe and Australia in January 2021 and was surprised how quickly its product was easily adopted. With more than 100 systems sold, the company proved that the market was ready for this innovation and is now expanding rapidly by partnering with leading orthopedic implant companies.
“This first surgery in the USA is an important milestone for Pixee Medical as the country represents 50% of the worldwide market and more than 800 000 TKR a year. Today, Knee+ is well suited to help ambulatory surgical centers (ASC) face the significant increase in knee surgery. These centers need intuitive, effective, attractive, and affordable solutions to meet their patient’s needs, now.” said Sébastien Henry, Founder and CEO of Pixee Medical.
“I have been impressed by the efficiency of the system. The product works well and flows nicely” commented Dr Antonia Chen, first surgeon to perform a Total Knee Arthroplasty using Knee+ augmented surgery in the USA. “The product doesn’t need a big equipment and offers an easy and efficient way to achieve a plan compared to robotics and other more traditional navigation systems. You can perform a TKR with only a small set of reusable instruments and essential information in your field of view. This is probably the most cost-effective solution for ASC”.