What To Know
- The HAART 200 Device simplifies bicuspid aortic valve repair by stabilizing the annulus and remodeling the valve into a symmetric, 180-degree leaflet orientation, eliminating the need for extensive dissection of the aortic root, and facilitating leaflet repair.
- With the commercial availability of the HAART 200 and HAART 300 Aortic Annuloplasty Devices, BioStable can offer surgeons within the European a comprehensive portfolio of aortic valve repair solutions that address all forms of aortic valve insufficiency.
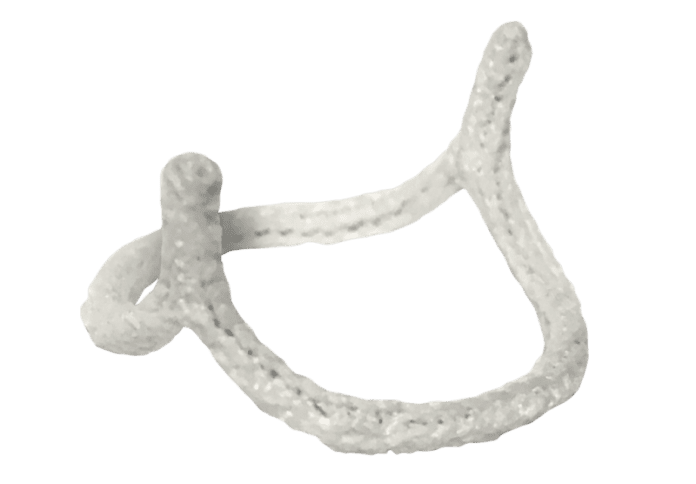
HAART 200 Aortic Annuloplasty Device
HAART 200 Aortic Annuloplasty Device first commercial implantation was performed on Monday, August 2nd by Professor Marek Jasinski, Chief of Cardiac Surgery at Wroclaw Medical University (Wroclaw, Poland). BioStable Science & Engineering announced the news today.
Additional procedures were completed on Thursday, August 5th by Professor Marek Deja, Chief of Cardiac Surgery at the Medical University of Silesia (Katowice, Poland). These two respected heart centers were the first selected to receive the device as part of the limited European commercial launch of the technology announced by the company in April.
Professor Jasinski commented, “The HAART 200 Aortic Annuloplasty Device is a great addition to our aortic valve repair tool kit providing a new option for the most common congenital heart defect, bicuspid aortic valve disease. Our experience with the HAART 300 Aortic Annuloplasty Devices in trileaflet aortic valve repair has shown us how the devices simplify aortic valve repair and at the same time provide durable annuloplasty. This allows us to expand the benefits of valve repair to more patients. The availability of the HAART 200 Device is critically important because patients with bicuspid aortic valve disease are younger, multiplying the benefits of valve repair vs. replacement.”
Although compelling long-term clinical data suggest that almost all bicuspid aortic valves should be repaired[1], current repair techniques are complex and not widely adopted. The HAART 200 Device simplifies bicuspid aortic valve repair by stabilizing the annulus and remodeling the valve into a symmetric, 180-degree leaflet orientation, eliminating the need for extensive dissection of the aortic root, and facilitating leaflet repair.
John Wheeler, President and CEO, concluded by saying, “BioStable is very pleased to initiate commercialization of the HAART 200 Aortic Annuloplasty Device alongside the HAART 300 Devices. With the commercial availability of the HAART 200 and HAART 300 Aortic Annuloplasty Devices, BioStable can offer surgeons within the European a comprehensive portfolio of aortic valve repair solutions that address all forms of aortic valve insufficiency.”