What To Know
- “Decades later, we are delighted to be recognized as a robotic surgery center of excellence, leaving another indelible mark as the first hospital in all of Europe to offer the Hugo RAS system and the first in the world to have Medtronic’s two RAS platforms — the Hugo system for soft tissue and the Mazor™ system for spinal surgery.
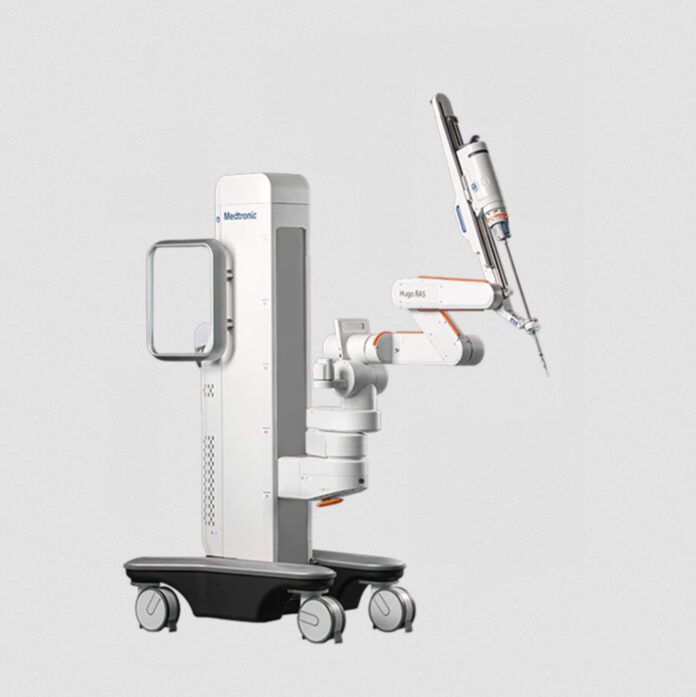
- The Hugo RAS system — Medtronic’s solution to historic cost and utilization barriers that have kept surgical robotics out of reach for many hospitals — is a modular, multi-quadrant platform designed for a broad range of soft-tissue procedures.
Hugo Robotic-assisted Surgery System: Today Medtronic plc (NYSE:MDT) and OLV Hospital Aalst today announced that the first clinical procedure in Europe was performed with the Hugo™ robotic-assisted surgery (RAS) system.
The robotic prostatectomy was performed by Prof. Alexandre Mottrie, M.D., head of urology at OLV Hospital in Aalst, Belgium, and chief executive officer and founder of the Belgium-based ORSI Academy, a multidisciplinary center for training, research and development, and data analysis to improve minimally invasive surgery best practices.
“Performing Europe’s very first procedure with the Hugo RAS system is a career highlight for me,” said Dr. Mottrie. “With more than two decades and 4,000 robotic-assisted surgery procedures under my belt, I am intimately aware of the barriers that have kept the benefits of surgical robotics from physicians, hospitals, and patients. Now, I believe we are entering a new era filled with greater access and flexibility.”
A form of minimally invasive surgery, robotic-assisted surgery offers fewer complications, shorter hospital stays, faster return to normal activities, and smaller scars than open surgery.1–3,†
“This is an exciting and important moment for healthcare in Europe and we’re proud to share it with Dr. Mottrie and the team at OLV,” said Megan Rosengarten, president of the Surgical Robotics business, which is part of the Medical Surgical Portfolio at Medtronic. “Dr. Mottrie has left a meaningful mark on our program over the many years we’ve worked together, and now, through our partnership with OLV, Medtronic’s journey to bring the benefits of robotic-assisted surgery to more patients in Europe is well underway.”
The Hugo RAS system — Medtronic’s solution to historic cost and utilization barriers that have kept surgical robotics out of reach for many hospitals — is a modular, multi-quadrant platform designed for a broad range of soft-tissue procedures. It combines wristed instruments, 3D visualization, and Touch Surgery™ Enterprise, a cloud-based surgical video capture and management solution, with dedicated support teams specializing in robotics program optimization, service, and training.
In 2021, Medtronic announced the first urologic and gynecologic procedures with the Hugo system in Latin America and Asia-Pacific. Those procedures and cases in Europe will become part of the Hugo RAS system patient registry, which is collecting clinical data to support regulatory submissions around the world.
“The Hugo RAS system introduces the long-awaited power of choice in the category and will redefine all that robotic-assisted surgery can make possible,” said Henk Westendorp, senior country director Benelux at Medtronic. “Medtronic thoughtfully designed the Hugo RAS system with surgeons in mind and patients at heart to tackle today’s barriers to adoption in a future-proofed way. We know that by innovating real solutions for the way surgeons want to work — alongside partners like OLV Hospital Aalst who share our passion for advancing patient care — we can make a substantial impact.”
“We’re incredibly proud to have left our stamp on medical history as the very first center in the region to embrace surgical robotics in 1999,” said Peter Verhulst, chief executive officer, OLV Hospital Aalst. “Decades later, we are delighted to be recognized as a robotic surgery center of excellence, leaving another indelible mark as the first hospital in all of Europe to offer the Hugo RAS system and the first in the world to have Medtronic’s two RAS platforms — the Hugo system for soft tissue and the Mazor™ system for spinal surgery.
The OLV Hospital closely monitors innovation in the medical world and often plays a pioneering role in the introduction of new minimally invasive techniques. The worldwide reputation of our OLV doctors in the field of robotic surgery and other minimally invasive procedures is a result of this. With the Hugo RAS system, we are again at the forefront, with the latest medical innovation that is designed with the patient at heart.”
The Hugo RAS system is commercially available in certain geographies. Regulatory requirements of individual countries and regions will determine approval, clearance, or market availability. In the EU, the Hugo RAS system is CE marked. In Canada, the Hugo RAS system has a medical device licence. The Hugo RAS system is approved in Australia. In the U.S., the Hugo RAS system is an investigational device not for sale. Touch Surgery Enterprise is not intended to direct surgery, or aid in diagnosis or treatment of a disease or condition. More on the Hugo Robotic-assisted Surgery System here.