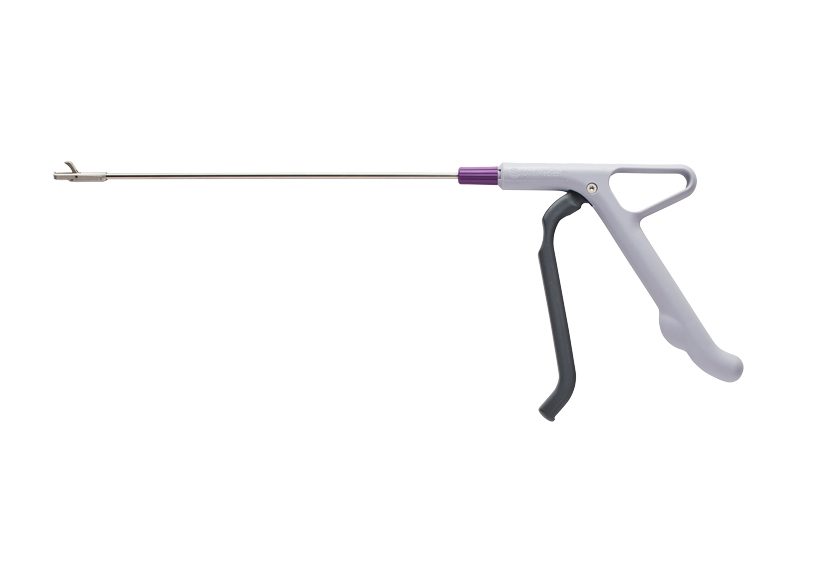
Innovia Medical (“Innovia”) is thrilled to announce the expansion of their single-use instrument product line into the U.S. The product leading the expansion is a gynecology product called the Cervical Rotating Biopsy Punch which supports urgently needed procedures like cancer diagnosis.
The single-use instrument was first introduced by DTR Medical Ltd (“DTR Medical”) in Wales in 2012 is now offered by Summit Medical LLC. (“Summit Medical”), a Minnesota based manufacturer of medical devices.
Since the initial development of the Cervical Rotating Biopsy Punch, the product has been used by numerous National Health Service (NHS) clinics and hospitals in the U.K. that have provided annual product feedback allowing for continuous product innovations. As part of an ongoing project with a leading Welsh University, a pioneering technique, known as biorefining, was used to recreate low carbon technologies for both the Cervical Rotating Biopsy Punch and it’s packaging. The instrument handle is now made of 20% renewably sourced propanediol (PDO) made from corn starch, making it both lightweight and recyclable. The polybag packaging was biorefined in 2020 to save 1,200 pounds of plastic each year. The Cervical Rotating Biopsy Punch has 2.8 times less of an environmental impact than other single-use products on the market like the metal Tischler Cervical Biopsy Punch and Baby Tischler Cervical Biopsy Punch.
Single-use instruments like the Cervical Rotating Biopsy Punch eliminate cross contamination, inadequate samples and repeat procedures. With Cervical cancer screenings down 68% in the U.S. due to COVID-19 [1], the importance of an adequate biopsy is essential in reducing the time to an accurate diagnosis.
“We believe introducing the Cervical Rotating Biopsy Punch to the U.S. is a natural expansion of our Single-Use Instrument category. Immediate benefits will improve the quality of patient care from acute care facilities to small offices that lack reprocessing environments. The quality of biopsy is notable and user feedback over the past decade has been extremely positive.” Said Marcus Super, Vice President of Marketing at Innovia Medical.