What To Know
- , a board-certified emergency physician and inventor says, “Many common life-saving procedures that have been done for decades in medical care have been severely restricted during the pandemic due to the danger of contaminating the healthcare providers who are performing these treatments.
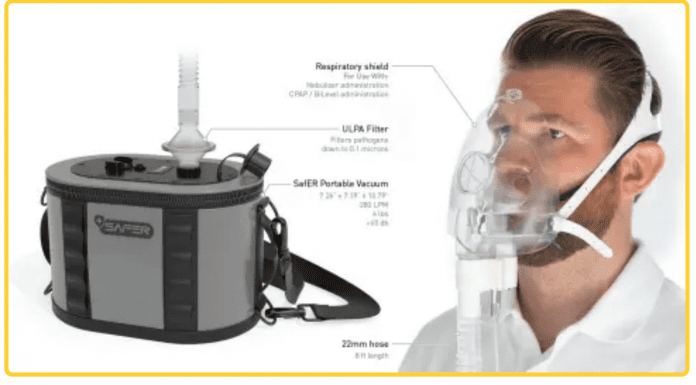
- Emergency physicians teamed with ROBRADY, a world-class product development firm, to create a portable negative pressure ULPA (Ultra Low Particulate Air) filtration system used by healthcare providers in any healthcare setting while administering patient care, lifesaving respiratory treatments and procedures or during patient transport.
Emergency physicians teamed with ROBRADY, a world-class product development firm, to create a portable negative pressure ULPA (Ultra Low Particulate Air) filtration system used by healthcare providers in any healthcare setting while administering patient care, lifesaving respiratory treatments and procedures or during patient transport.
This innovative system utilizes an ultra-quiet negative pressure generator weighing less than 7 pounds with a family of attachments turning any emergency or non-emergency healthcare setting into a negative pressure isolation environment. The system adds an additional layer of protection for the healthcare provider against airborne contaminants produced during common treatments and life-saving aerosol-generating procedures, such as nebulizers, continuous positive airway ventilation (CPAP), intubation, and more.
Rick Blubaugh, DO., a board-certified emergency physician and inventor says, “Many common life-saving procedures that have been done for decades in medical care have been severely restricted during the pandemic due to the danger of contaminating the healthcare providers who are performing these treatments. We could not stand by and watch without doing something that changed the situation for our patients and colleagues. This allows us to get back to safely delivering that care when it is needed most.” While the system from SafER Medical Products, LLC is COVID-inspired, it can and should be used to help mitigate exposure from any airborne pathogens that healthcare personnel are routinely exposed to.
The system is FDA listed and in use by several hospitals and EMS agencies. Research paths are underway with physicians and scientists from Oklahoma State University, University of Missouri, Massachusetts College of Pharmacy and Health Sciences, and the National Physical Laboratory in the UK. Dr. Blubaugh sees the SafER system becoming the new standard of care for aerosol-generating procedures and “the Latex glove of the 21st century”. The SafER and ROBRADY team work around the clock to produce and deliver the system across the country and soon to distribute this life-saving protection globally.