What To Know
- He holds a BA degree from The University of Notre Dame and an MBA from Johns Hopkins University and also served as a Lieutenant in the United States Navy.
- In a case report that was published in The Cureus Journal of Medical Science in April 2021, it was documented that a traditional external ventricular drain (EVD) failed during the….
IRRAS, an emerging leader in delivering innovative products for neurocritical care, today announced that Will Martin has formally assumed the role of President and Chief Executive Officer (CEO). This promotion comes as part of a planned succession process that was first announced in April 2021. Kleanthis G. Xanthopoulos, Ph.D., the company’s CEO for the previous 6 years, will remain on the Company’s Board of Directors.
This leadership transition comes at an important point in the company’s growth. After showing key progress across the company in recent years, IRRAS is now focused on driving commercial adoption and accelerating revenue growth, and Mr. Martin’s commercial experience is being tapped to lead the company into this exciting period of accelerating growth.
“We have a strong foundation in place to support future growth, and I’m incredibly excited to lead IRRAS during this important period,” said Martin. “Our company now has key regulatory clearances in place for its products, and we recently opened our first in-house manufacturing facility. As COVID restrictions lessen globally, our products are helping more critically ill patients each day, and I look forward to positioning the company to best support hospitals and investors around the world.”
Mr. Martin joined IRRAS as Chief Commercial Officer in 2018 before assuming the role of President in June 2020. Prior to IRRAS, he served as General Manager of the Peripheral Vascular devices business for Philips Healthcare after its acquisition of Volcano. Under his leadership, the division was one of the fastest growing across all of Philips, and he played a key role in the acquisition of Spectranetics for more than $2B. Prior to that role, Mr. Martin was the Vice President of Commercial Operations at AtheroMed, Inc. which was acquired by Volcano Corporation for a total consideration of approximately $160M. He holds a BA degree from The University of Notre Dame and an MBA from Johns Hopkins University and also served as a Lieutenant in the United States Navy.
IRRAS is focused on designing, developing, and commercializing innovative solutions that transform fluid management after surgical procedures where drainage is required. Traditional cerebrospinal fluid (CSF) management systems, such as external ventricular drains (EVDs), help control a patient’s intracranial pressure (ICP) after hemorrhagic stroke or traumatic brain injury. Unfortunately, EVDs rely on the same concept of gravity-driven drainage that was first performed over 275 years ago. This dated approach is plagued by catheter occlusions and infection which lead to inefficient drainage, negative outcomes, and increased overall treatment cost. On the other hand, IRRAflow, is a next-generation technology to treat intracranial pathologies that has been cleared for commercial release in the United States and the European Union.
IRRAflow’s unique mechanism of action addresses the complications associated with the current treatment methods by using a dual-lumen catheter that combines automated irrigation, controlled drainage, and continuous intracranial pressure (ICP) monitoring into one simple system. This combination of irrigation and drainage results in a process known as active fluid exchange. Active fluid exchanges ensures that drainage holes remain blockage free and helps replace the collected toxic material with a neutral irrigation solution, resulting in more efficient removal than relying upon gravity alone.
Building upon this clear need for new innovative tools, IRRAS has reported three consecutive quarters of revenue growth as it has navigated the impact of the Covid-19 global pandemic. With its commercial activity increasing, the company stated that it has treated approximately 250 patients with its IRRAflow system at more than 30 leading institutions. At the end of the first quarter of this year, 84 IRRAflow systems were placed with customers in 20 countries across 4 continents, and patient treatments had again exceeded pre-pandemic levels.
During the first quarter of 2020, the company reported that it finalized commercial agreements with two key comprehensive stroke centers in the US, Buffalo General Medical Center, and West Virginia University Hospital. The partnerships quickly generated positive data for the company as the team at Buffalo General, led by Dr. Elad Levy and Dr. Adnan Siddiqui, recently published their initial case studies regarding their use of IRRAflow.
In a case report that was published in The Cureus Journal of Medical Science in April 2021, it was documented that a traditional external ventricular drain (EVD) failed during the treatment of an intraventricular hemorrhage (IVH). The failed EVD was then replaced by an IRRAflow, which ultimately cleared the patient’s ventricular space of blood. In this manuscript, the surgeons noted that “our case demonstrates clear radiographic and clinical superiority of the IRRAflow system compared to standard EVD.”
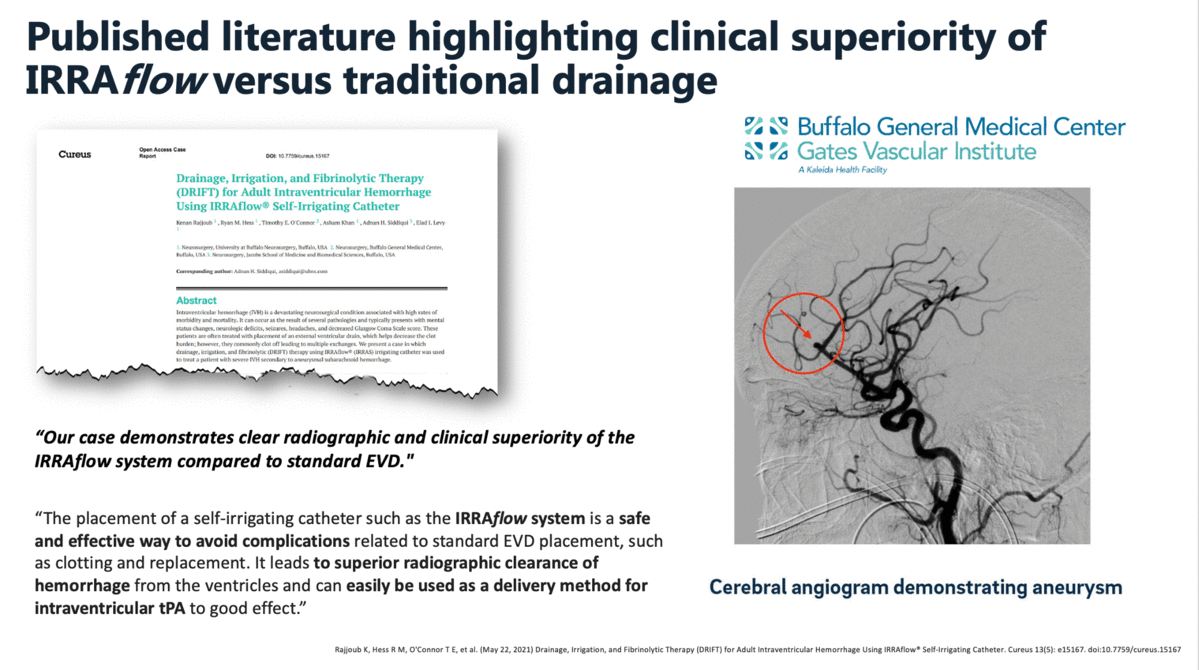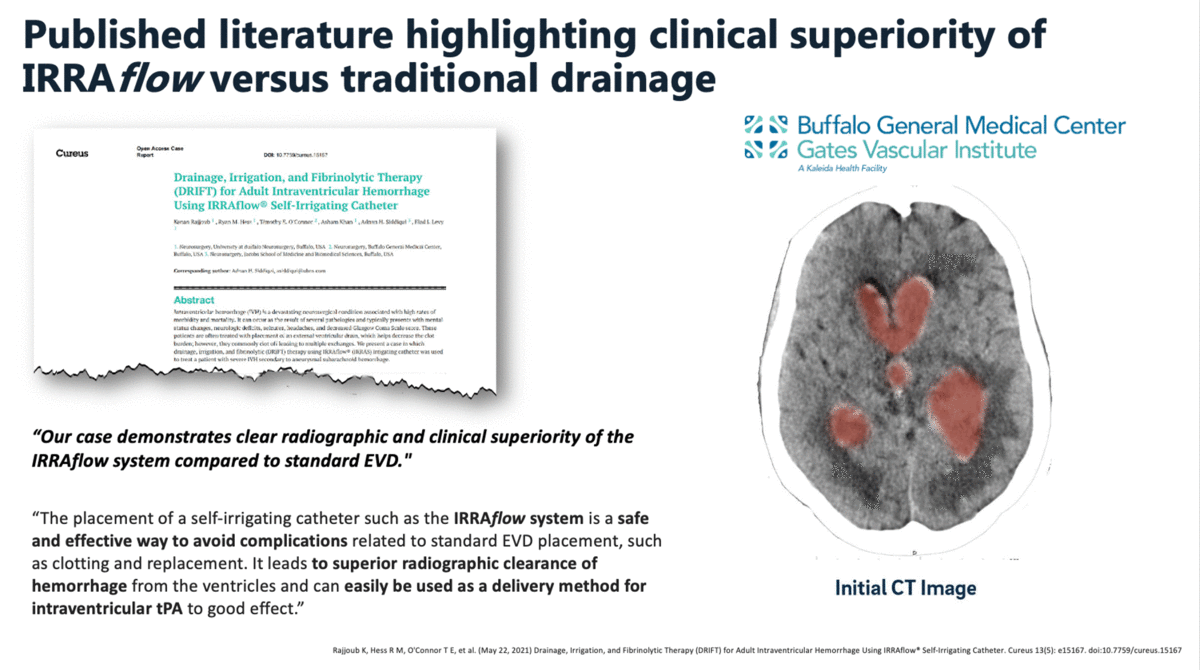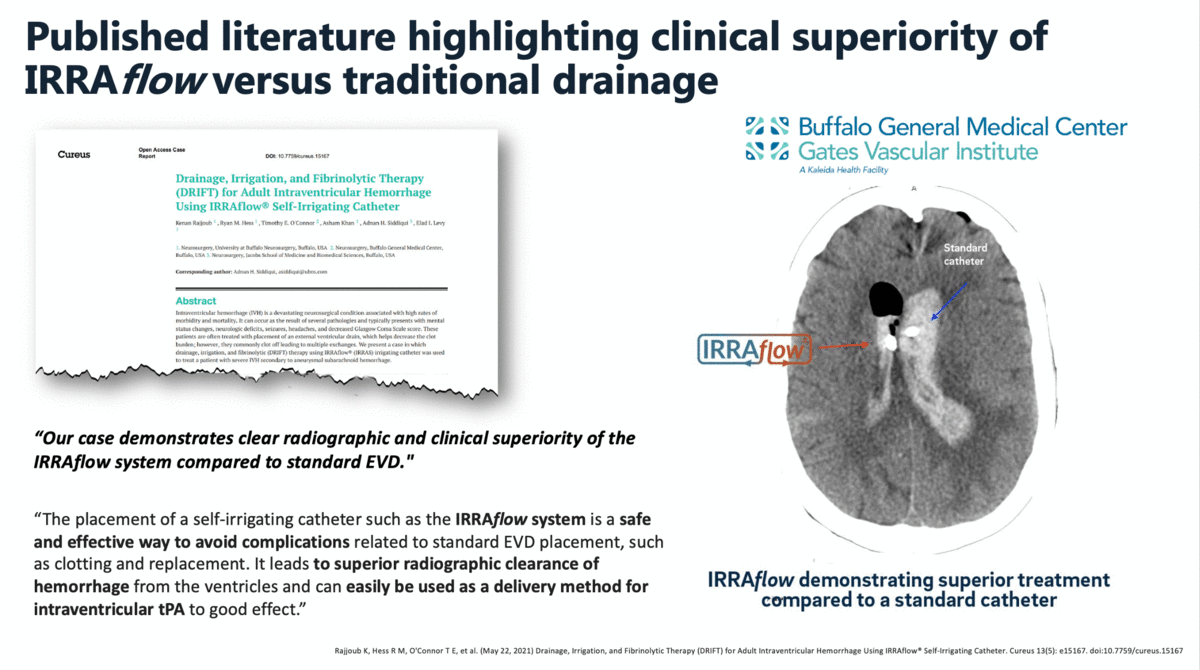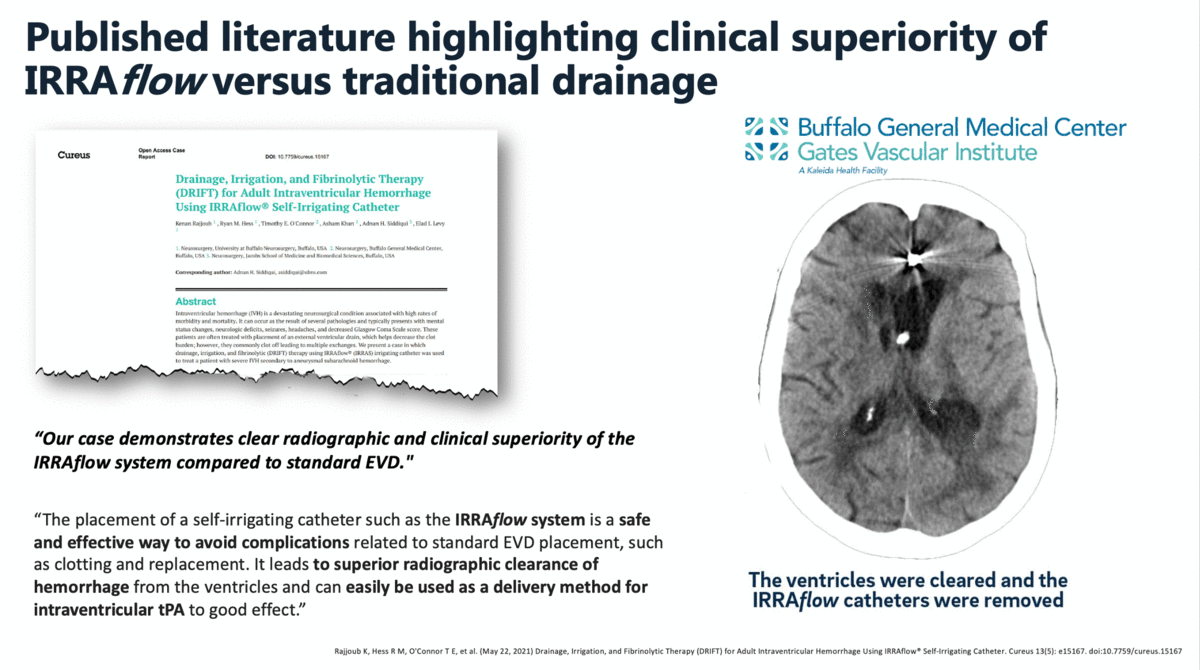
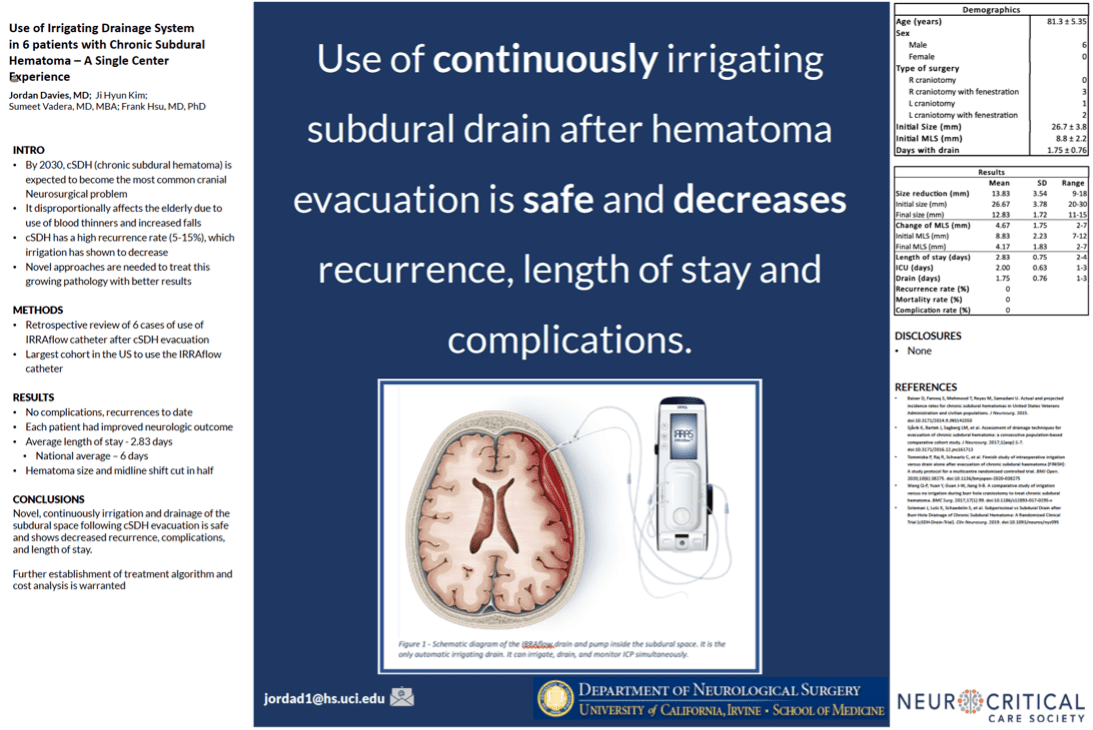
Additional clinical data presented by surgeons from the University of California – Irvine (UCI) at the 2019 Annual Meeting of the Neurocritical Care Society highlighted the effectiveness and safety of IRRAflow in treating chronic subdural hematomas as well. This poster presentation highlighted a 0% recurrence rate with IRRAflow as well as a greater than 50% reduction in hospital length of stay from an average of 6 days to 2.83 days.
As normal activities resume, the company is excited to build upon this impactful clinical performance of its products. The IRRAflow product launch is accelerating globally, especially as US adoption increases and global markets reopen with increasing vaccination rates, and the company reports that it is currently seeking European approval for its Hummingbird product line. The company is also investing in multiple clinical trials to further confirm the clinical superiority of its IRRAflow system.
IRRAS’ operations are based in San Diego, California, with additional offices located in Munich and Stockholm. IRRAS markets and sells its innovative IRRAflow and Hummingbird ICP Monitoring product lines to hospitals worldwide through its direct sales organization in the United States, Germany, and the Nordic region, as well as an international network of distribution partners.