LayerBio, Inc. (LayerBio), an ophthalmology therapeutics company, announced today that the United States Patent and Trademark Office (USPTO) has issued US Patent No. 11,185,441.
“This patent issuance marks an important milestone for the OcuRing platform and recognition of novelty and uniqueness of our drug delivery technology,” said Kenneth Mandell, M.D., Ph.D., CEO and Founder of LayerBio. “LayerBio will continue to develop our portfolio of patents for the OcuRing platform in order to produce safer, more effective and better-tolerated alternatives to topical eye drops for patients undergoing cataract surgery.”
The patent provides coverage for a variety of drug product compositions, methods of use, and delivery systems for LayerBio’s propriety bioerodible drug delivery rings that release therapeutic medications for use in cataract surgery.
“OcuRing has the potential to transform the standard of care for cataract surgery,” said Dr. Richard Lindstrom, M.D., a leading expert in cataract surgery and member of LayerBio’s Board of Directors. “By automatically releasing medicine inside the eye after cataract surgery, OcuRing™ can ensure compliance, improve outcomes and enhance the overall patient experience.”
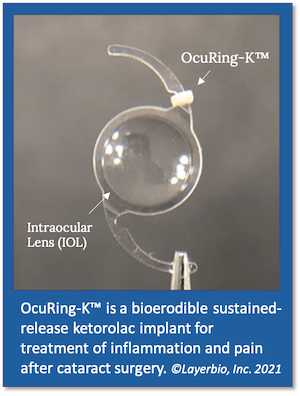
LayerBio’s lead product candidate OcuRing-K™ is a sustained-release nonsteroidal anti-inflammatory drug (NSAID) formulation of ketorolac, an opioid-sparing analgesic with proven efficacy for reducing inflammation and pain associated with cataract surgery.
“OcuRing-K is a game-changer because it provides all of the benefits of an NSAID without the ocular surface side effects,” said Dr. Eric Donnenfeld, M.D., chair of LayerBio’s medical advisory board. “By delivering ketorolac directly inside the eye, it maintains a therapeutic plateau and essentially eliminates the risks of surface corneal toxicity associated with NSAID eye drops.”
In addition to NSAIDs, the OcuRing™ platform has the capacity to deliver other ophthalmic medications, including corticosteroids, antibiotics and glaucoma medications. LayerBio will continue to develop innovative products that address unmet medical needs for cataract surgery and other eye conditions.