What To Know
- An Example Of A Colonic Lesion, Or Polyp, With A Most Likely Prediction Of A Nice (Nbi International Colorectal Endoscopic) Type 2 Adenoma As Seen In Near Focus Under The White Light And Nbi (Narrow Band Imaging) Observation Modes Of A Cf-Hq190L Colonovideoscope.
- In addition to the current indications for NBI, Olympus has received FDA clearance for the screening and surveillance of Barrett’s esophagus and is currently exploring other claims for NBI both in gynecology and general surgery.
March 15, 2021
Narrow band imaging (NBI): New evidence supports use of NBI to characterize polyps found during screening colonoscopies for colorectal cancer.
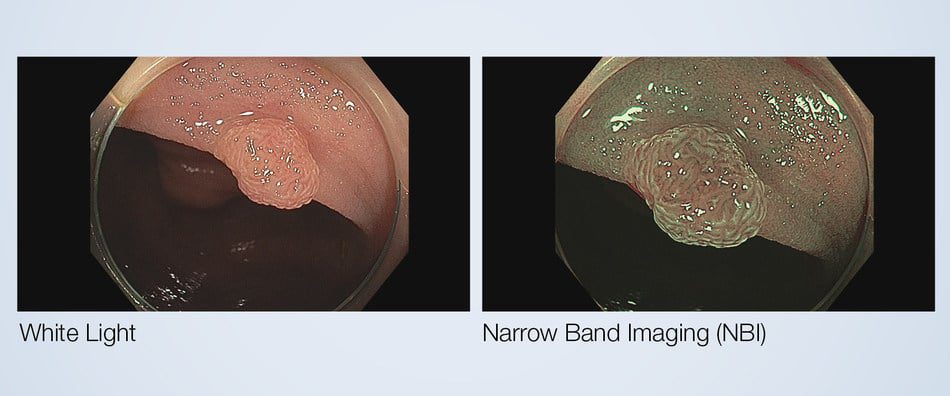
By applying the NBI International Colorectal Endoscopic (NICE) classification during a screening colonoscopy, physicians can make high-confidence predictions of histology for polyps 5mm or smaller, known as diminutive polyps. Patients and healthcare systems can benefit from implementation of NBI as a decision-making support tool.
The data submitted to the FDA in support of the 510(k) clearance included a meta-analysis of prospective real-time clinical studies of NBI use during colonoscopy.1 The data show experienced endoscopists employing a validated classification system, such as the NICE classification, have:
- Demonstrated 93% sensitivity (89-96%, 95% Confidence Interval, 59-98% range) in predicting adenomatous histology of diminutive polyps during colonoscopy when made with high confidence.
- Demonstrated 85% specificity (74-92%, 95% Confidence Interval, 44-99% range) in predicting adenomatous histology of diminutive polyps during colonoscopy when made with high confidence.
- Demonstrated greater than 90% Negative Predictive Value in predicting adenomatous histology of diminutive polyps during colonoscopy when made with high confidence.
- Achieved greater than 90% agreement with pathological analysis in assigning post-polypectomy patient surveillance intervals following colonoscopy.
- Were more likely to make high-confidence predictions of diminutive polyp histology when using Near Focus mode colonoscopes than those using standard focus colonoscopes in a randomized clinical trial.
Typically, polyps are detected and removed during colonoscopy and sent out for pathological diagnosis. Given the abundance of diminutive colorectal polyps, the American Society for Gastrointestinal Endoscopy (ASGE) recognized the importance of making real-time predictions of polyp histology. ASGE created a Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI) initiative to assess technologies for the ability to aid physicians in the characterization of polyps. Of the technologies evaluated by ASGE’s PIVI initiative, NBI was the only one shown to meet the performance criteria for incorporation in clinical practice.2
“The science behind the practice of resecting and discarding diminutive polyps identified using NBI is established,” said Douglas Rex, M.D. Distinguished Professor at the Indiana University School of Medicine and Director of Endoscopy at Indiana University Hospital. “However, its implementation in practice has been impeded by various factors. Despite this, experts find value in interrogating lesions with NBI.” Such applications include deciding whether to request pathology recuts of diminutive high-confidence adenomas deemed normal by pathology, selecting NICE type 1 lesions for endoscopic mucosal resection (EMR) and cold EMR, and facilitating accurate identification of recurrence on EMR.3
“We are excited about these newly cleared indications for using NBI to support colorectal cancer screening,” said Kevin Mancini, Vice President for Endoscopy at Olympus America, Inc. “NBI is an important tool, available as standard on all Olympus colonoscopes, which can be used by physicians to assist in decision making with the goal of improving patient care.”
NBI is an optical imaging technology that enhances the visibility and contrast of vessels and surface patterns on the mucosa. Unlike white light, which uses all colors in the spectrum, NBI filters out all but blue and green light which are absorbed by hemoglobin and penetrate only the surface of human tissue. NBI also has clinical applications in urology, pulmonology and rhinolaryngology (ENT). In addition to the current indications for NBI, Olympus has received FDA clearance for the screening and surveillance of Barrett’s esophagus and is currently exploring other claims for NBI both in gynecology and general surgery.