What To Know
- The NuSpec is designed to work on most patients, reducing a clinic’s need to carry an extensive range of speculum styles and sizes.
- The Nella VuSleeve, an easy-to-apply clear sheath that provides lateral sidewall retraction and the Nella VuLight, a single use LED illuminator for superior illumination, both can be used with most reusable and disposable vaginal specula.
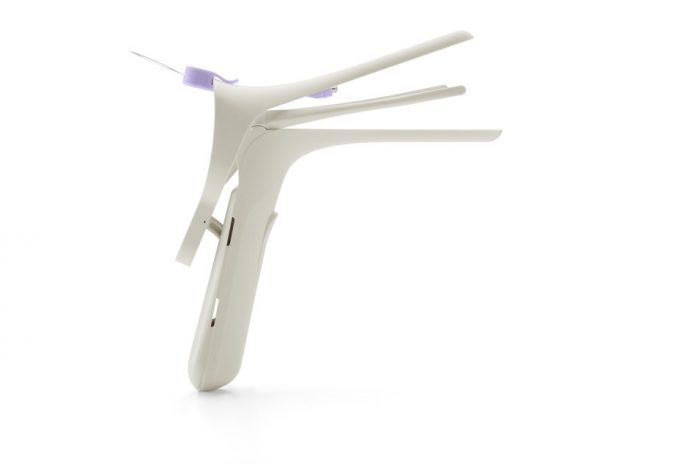
Nella NuSpec, a smarter reusable vaginal speculum was introduced today by Ceek Women’s Health.
Nella NuSpec was developed in partnership with OBGYN providers and patients through an extensive 5-year R&D process. The reusable vaginal speculum commonly used today is made from metal and has not changed much from those developed over 150 years ago. The NuSpec is the first major redesign of the reusable vaginal speculum that takes into account both the patient experience along with unmet provider needs.
Speculum insertion can be uncomfortable for many patients but the NuSpec utilizes a narrow bill—about the size of a regular tampon– to allow for comfortable insertion. Once opened, lateral retractors retain vaginal sidewalls so providers have adequate visibility and access to the cervix. Nella NuSpec’s medical-grade polymer is temperature neutral, eliminating the jarring cold sensation for patients, and does not require lubrication, reducing the risk of compromised test results. The NuSpec is designed to work on most patients, reducing a clinic’s need to carry an extensive range of speculum styles and sizes. It is cleaned and sterilized with the same methods used for current metal specula without requiring disassembly.
The Nella NuSpec was successfully tested in a randomized IRB-approved clinical study on women with different body types and across life stages. Because it is a reusable vaginal speculum, the carbon footprint of the NuSpec is also notably lower than existing single-use plastic specula.
“I am very excited to introduce the Nella NuSpec to patients and their providers. The OBGYNs are tough and will not settle for a suboptimal product. Our clinician advisors have reviewed hundreds of prototypes, evaluating every detail. This device will be game-changing in the care patients receive,” said Fahti Khosrowshahi, CEO and founder of Ceek Women’s Health.
In the U.S. alone, more than 55,000 clinicians use a speculum on average 7-30 times per day, and cumulatively more than 60,000,000 times annually. The Nella NuSpec improves comfort for any patient, but it can make a drastic difference in exam experience for specific populations, such as first-timers, transgender patients, rape or trauma patients, post-menopausal women, and cancer survivors.
“As an OBGYN, we have a responsibility to provide exceptional care and positive experiences for our patients. The Nella line is a brilliant new offering that helps OBGYNs manage exams with more personalization, which is long overdue in the women’s health market,” states Dr. Jessica Shepherd, OBGYN and Ceek Women’s Health Ambassador. “The NuSpec helps me improve the quality of care my patients receive and provides a tool for increased dialogue regarding their exam experience.”
The NuSpec Reusable Vaginal Speculum is part of the Nella line of products designed by Ceek Women’s Health which also includes two accessory products. The Nella VuSleeve, an easy-to-apply clear sheath that provides lateral sidewall retraction and the Nella VuLight, a single use LED illuminator for superior illumination, both can be used with most reusable and disposable vaginal specula.