What To Know
- “The combination of the PROPEL Mini and the Straight Delivery System in one package is reflective of Intersect ENT's ongoing commitment to supporting the ENT community,” states Roheen Raithatha, MD, practicing Rhinologist at ENT and Allergy Associates and faculty member at Mt Sinai Hospital in New York.
- West, President and CEO of Intersect ENT, said, “Intersect ENT is pleased to provide our physicians with a complete package of PROPEL Mini and the Straight Delivery System.
The combined packaging of the SDS with PROPEL Mini received premarket approval by the U.S. Food and Drug Administration which follows PMA for the Straight Delivery System received in July 2020.
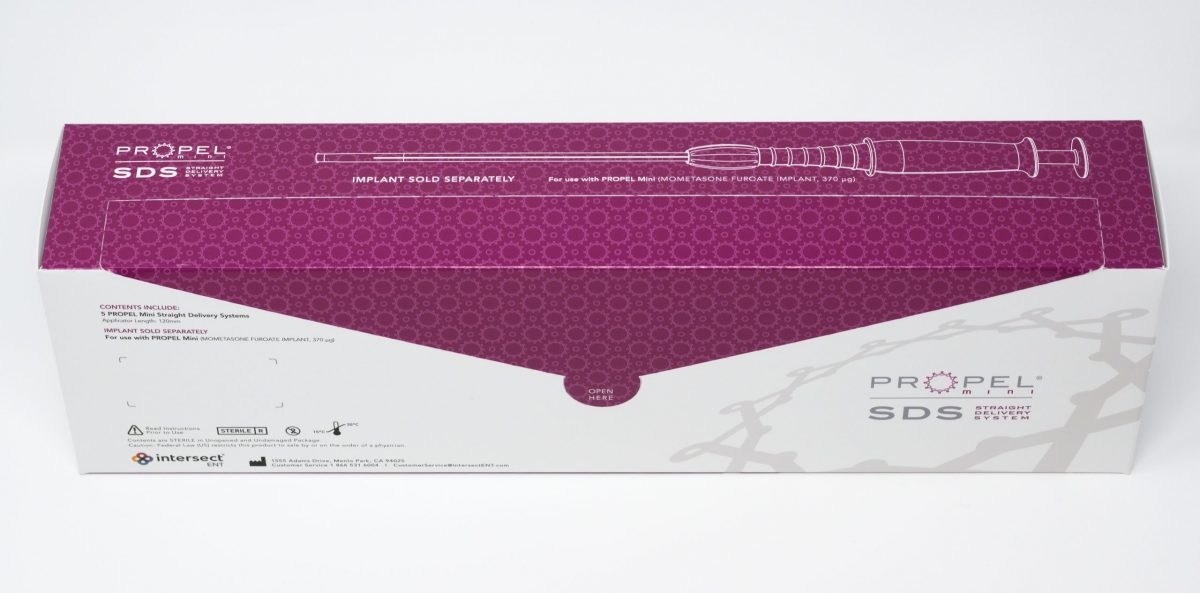
The Straight Delivery System is an extension of the PROPEL family of implants. It is specifically engineered for precise, consistent and easy delivery of the PROPEL Mini implant into the ethmoid sinus for maximum tissue apposition. The original curved delivery system will continue to be available with the PROPEL Mini sinus implant, offering physicians a suite of options when using PROPEL Mini following sinus surgery.
Thomas A. West, President and CEO of Intersect ENT, said, “Intersect ENT is pleased to provide our physicians with a complete package of PROPEL Mini and the Straight Delivery System. We are dedicated to responding to our customers’ feedback and providing them with the best combination of products to advance care for chronic sinusitis patients in various centers of care.”
“The combination of the PROPEL Mini and the Straight Delivery System in one package is reflective of Intersect ENT’s ongoing commitment to supporting the ENT community,” states Roheen Raithatha, MD, practicing Rhinologist at ENT and Allergy Associates and faculty member at Mt Sinai Hospital in New York. “I look forward to utilizing this newly bundled implant and delivery system during my FESS procedures.”
More on CNS coding here.

