HeartFlow, Inc., today announced that the National Institute of Health and Care Excellence (NICE) has renewed its support for the use of its AI-powered HeartFlow® FFRct Analysis to fight coronary heart disease (CHD), one of the leading causes of death in the UK.
View video here.
NICE has found that the HeartFlow Analysis continues to improve patient experience and reduce the need for invasive angiography, four years after its initial recommendation that the technology be used to support CHD diagnosis within the NHS. Furthermore, it has estimated that using the HeartFlow Analysis represents a cost saving of £391 per patient to NHS England, £177 higher than original calculations suggested in 2017.
The latest guidance represents a win for the use of digital technologies in the NHS and reflects its commitment to improving patient care through innovation. The HeartFlow Analysis was recently selected as one of the innovations supported by NHS England’s new MedTech Funding Mandate, which aims to provide new medical devices and digital products to patients faster.
NICE also recognizes the non-invasive technology’s improved performance in recent years, including:
- Ongoing incremental software releases to address security updates, compatibility updates and user experience improvements based on clinician feedback;
- A reduced turnaround time for sharing findings of the Analysis with clinicians – from 48 hours in 2017 to six hours;
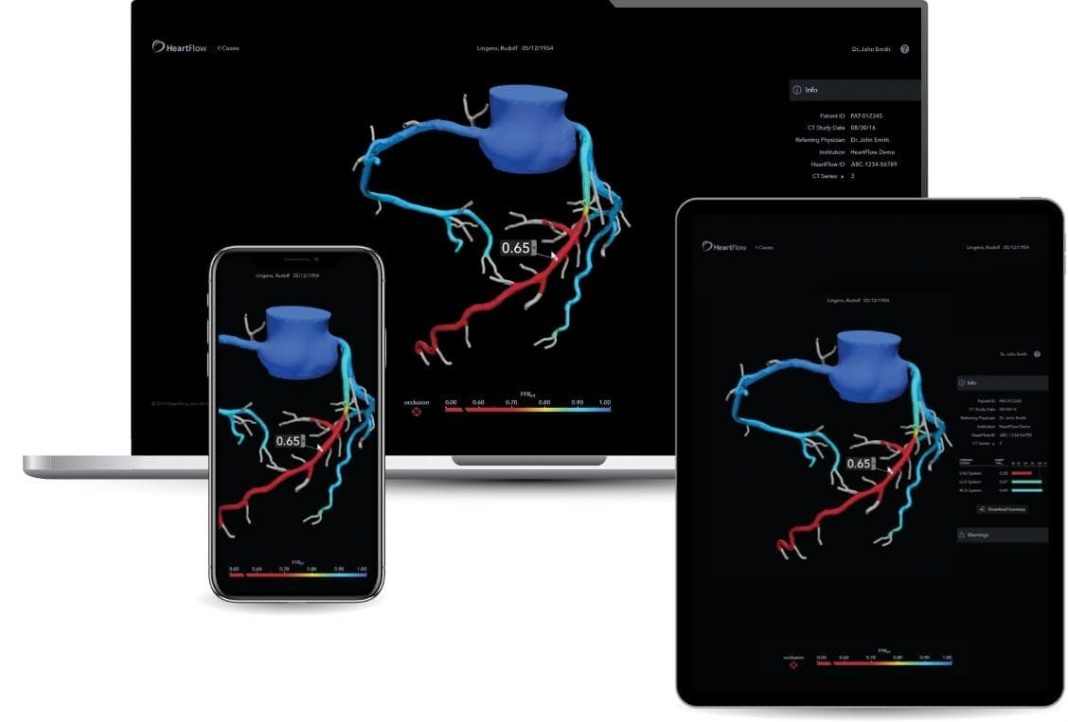
- Enhanced options for viewing the 3D model, such as via mobile platforms, integration with electronic health records and shared patient management updates.
Dr. Tim Fairbairn, Consultant Cardiologist, at Liverpool Heart & Chest Hospital and honorary senior lecturer at the University of Liverpool Hospitals says: “This latest assessment from NICE that the HeartFlow technology offers significant cost savings through avoidance of invasive investigations and streamlining treatment for patients reinforces what we have been seeing in clinical practice. In light of that, we’re pleased to continue its use within the healthcare service at Liverpool Heart and Chest Hospital where it is helping improve our care of CHD patients.”
The HeartFlow Analysis streamlines the diagnostic experience for patients – often enabling physicians to eliminate invasive diagnostic procedures for those who do not need them. It also helps physicians swiftly and accurately diagnose those who do need invasive procedures. The technology limits redundant non-invasive diagnostic testing, reduces patient time in hospital and face-to-face clinical contact, and helps ensure that hospital visits for those who do need them are streamlined, which is particularly crucial during the Covid-19 pandemic.
Dr. Campbell Rogers, Executive Vice President and Chief Medical Officer, HeartFlow, says: “It’s fantastic to see the renewed guidance from NICE which continues to advocate a CTA-first approach to diagnosing CHD. The HeartFlow Analysis has been identified as a best practice, non-invasive option for patients with stable, recent onset chest pain. It helps clinicians to make a diagnosis more confidently while reducing the time to diagnosis for patients.”
“The HeartFlow Analysis is now available in 65 NHS hospitals across England and is planned to be in 30 more by 2022. With the support of NICE and as part of NHSE’s MedTech Funding Mandate, we’re now in a position to scale and ensure that more clinicians and patients can benefit from our non-invasive technology and receive a quicker CHD diagnosis and treatment plan.