Northwell Health has been selected to join the Diabetic Foot Consortium as a satellite site and awarded a grant by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
The Diabetic Foot Consortium is led by a network of seven universities with satellite locations all working to drive clinical research. Northwell will serve as a satellite program and collaborate directly with the University of Michigan team. The NIDDK is part of the National Institutes of Health (NIH), the nation’s medical research agency.
Northwell’s $50,000 NIH grant will go toward refining wound care best practices and equity of care in the diabetic population. An estimated 37 million Americans are living with diabetes – many of them undiagnosed – and diabetic foot disease is a key factor that accounts for a measurable reduction in life expectancy.
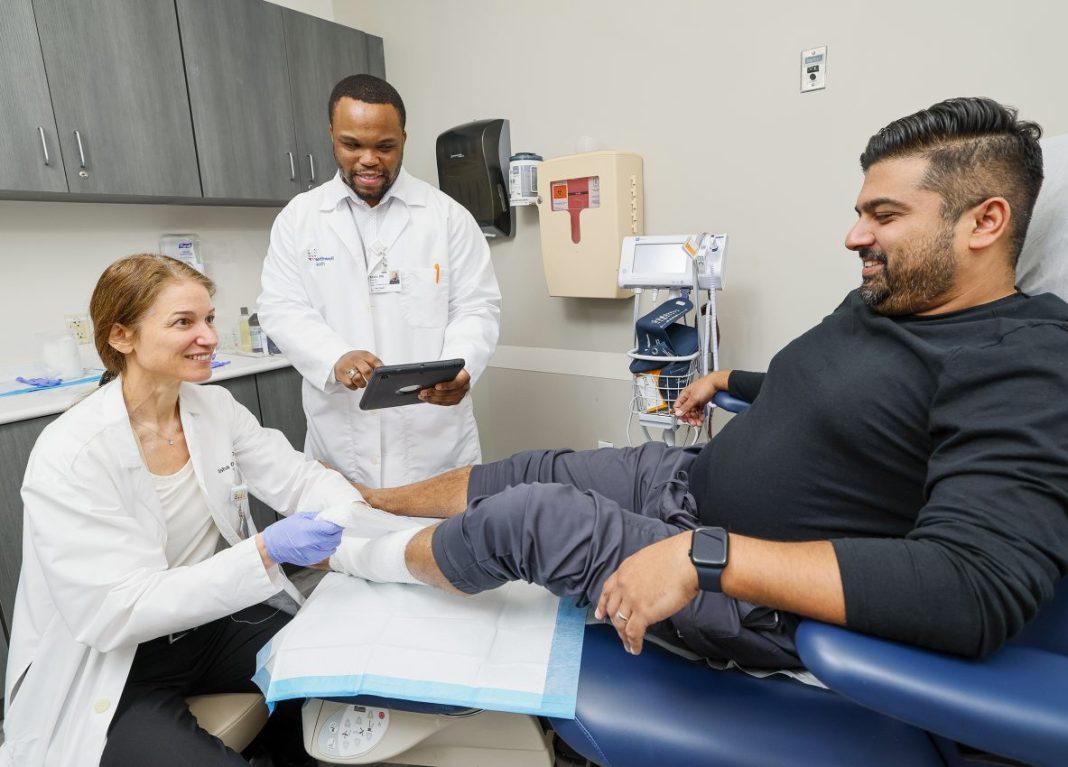
“Diabetic foot ulcers are the leading cause of lower limb amputations in the United States today, which is why we’re excited to be part of the solution to such a chronic health problem,” said Alisha Oropallo, MD, a vascular surgeon and director of the Comprehensive Wound Healing Center and Hyperbarics at Northwell Health. “As a satellite member of the Diabetic Foot Consortium, Northwell will be able to conduct studies and tap into research to improve the lives of New Yorkers living with diabetic foot ulcers.”
Northwell’s Comprehensive Wound Healing Center and Hyperbarics, located at 1999 Marcus Ave., Suite M6, in Lake Success, NY, specializes in diabetic wound care. The center features a state-of-the-art vascular lab, a New York State-licensed tissue bank and diagnostic imaging that can assess microperfusion.
For more information or to make an appointment, call 516-233-3780.