What To Know
- ChiroKlip joins the Osteotec Silicone Finger and Concentric Bone Graft System as a new member of the range of high-quality, Osteotec products available in the USA market within the orthopedic space.
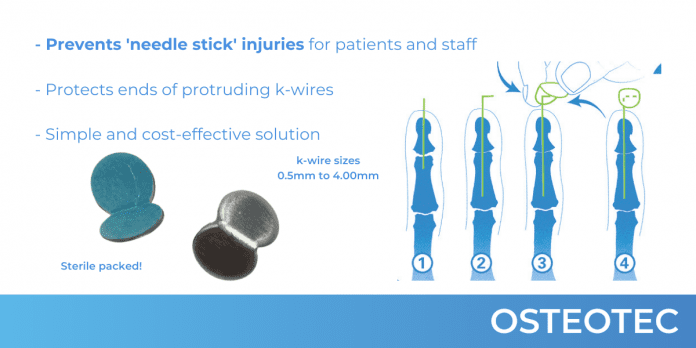
- ChiroKlip is a new concept in solving the perennial problem of protecting the protruding ends of K-wires and is a powerful self-fixing disposable device developed in the UK.
Osteotec Limited has announced that they have obtained FDA registration for ChiroKlip, the powerful self-fixing disposable device that solves the problem of protecting the protruding ends of K-wires.
ChiroKlip joins the Osteotec Silicone Finger and Concentric Bone Graft System as a new member of the range of high-quality, Osteotec products available in the USA market within the orthopedic space.
Osteotec is working to secure a distribution network for ChiroKlip across the US, as well as continuing to successfully expand in areas outside of the USA.
What is ChiroKlip?
ChiroKlip is a new concept in solving the perennial problem of protecting the protruding ends of K-wires and is a powerful self-fixing disposable device developed in the UK.
The butterfly-shaped clip is simple to use and protects the exposed end of the K-wire without using old-fashioned and inefficient corks or strapping, or complicated instrumentation.
Protecting the end of the K-wire with ChiroKlip can help prevent trauma or tearing of clothing or other items. The single-use ChiroKlip also helps in the removal of wires by allowing an excellent purchase on the wire.
ChiroKlip is widely used within UK hospitals and has a growing export market. Available in easy-to-use boxes of 25 packs, with each pack containing 2 gamma sterilized ChiroKlips.
“FDA registration for ChiroKlip will mean that all Osteotec own-manufactured devices can be sold in the USA, presenting a great opportunity for our export business,” said Andrew Dubowski, Osteotec Commercial Director.
“Osteotec products offer excellent commercial opportunities for orthopedic sales organizations in the USA. Adding our devices to your portfolio is a great way to offer a comprehensive solution to surgeons.”
Matt Woods, Osteotec Managing Director, said: “ChiroKlip is a fantastic addition to the Osteotec family, complementing and strengthening our existing product line. This is the latest acquisition Osteotec has made as we continue to grow our international offering.”