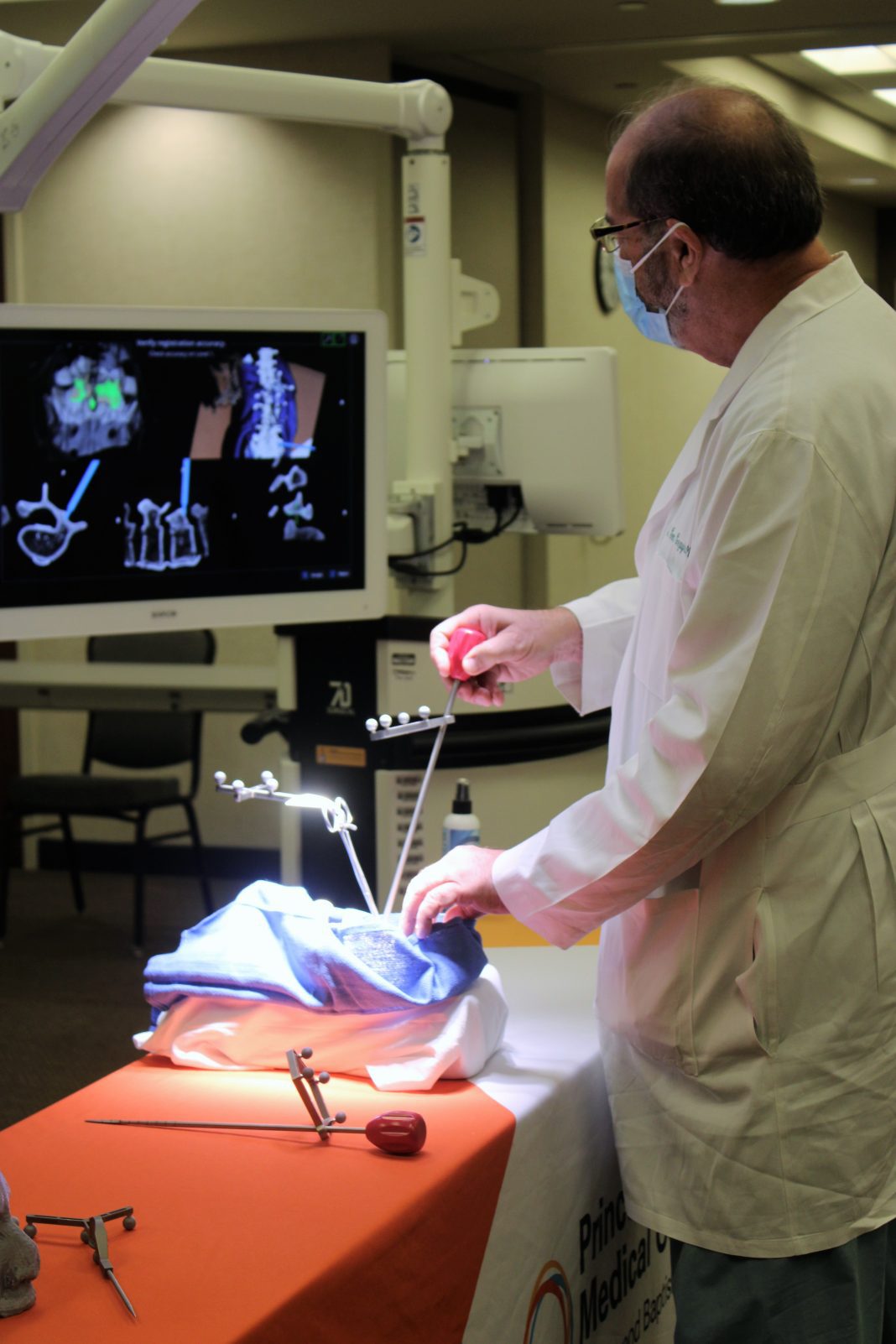
As hospitals around the world look for new and innovative ways to improve surgical outcomes and patient safety, Princeton Baptist Medical Center has taken a leap into the future with the installation of the 7D Surgical FLASH™ Navigation System for spinal and cranial procedures by being the first hospital in Alabama to deploy the technology.
This system virtually replaces standard fluoroscopy, providing the surgical team with a fast, accurate and radiation-free tool for the placement of spinal implants.
“Princeton Baptist Medical Center has always been proactive in ensuring that we have the right tools to protect our patients and enhance neurosurgical patient care, which is why we supported the purchase of the 7D Surgical FLASH Navigation System,” said Mike Neuendorf, Princeton CEO. “This important investment underscores our commitment to patient care and the communities we serve, for both advanced spinal and cranial procedures.”
The 7D Surgical FLASH Navigation System is the first and only platform that utilizes Machine-Vision Image Guided Surgery (MvIGS) technology. For the first-time, spine surgeons can guide their tools to the critical anatomy using sophisticated camera technology linked to a computer in the operating theater. The underlying technology is similar to what is used in the latest self-driving automobiles. Unlike time-consuming conventional image guided surgery (IGS) systems that depend on intraoperative radiation, this new MvIGS technology can achieve an incredibly fast surgical workflow for spine procedures, reducing operative time for patients and eliminating unnecessary radiation exposure.
“As a Neurosurgeon, the FLASH Navigation System technology provides me with enhanced precision when placing hardware in the spine, because I can see the anatomy in 3D,” said Resit Cezayirli M.D., an affiliated neurosurgeon at Princeton Baptist Medical Center. “I am proud to offer my patients the latest technology in spine surgery while eliminating unnecessary radiation still used in many hospitals.”