What To Know
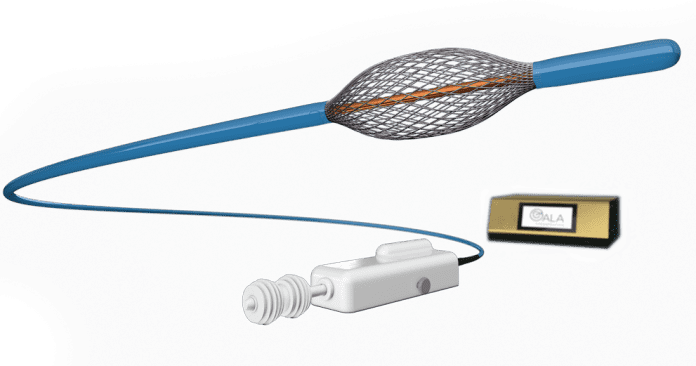
- RheOx System is designed to deliver non-thermal pulsed energy to the airways in the lungs to reduce mucus-producing cells, thereby improving the cough and mucus symptoms of chronic bronchitis.
- Our collaboration with the COPD Foundation to bring forward the CAT score as a meaningful patient-reported measurement helped enable a study design centered on an outcome reflecting the patient’s experience,”.
February 22, 2021
RheOx System is designed to deliver non-thermal pulsed energy to the airways in the lungs to reduce mucus-producing cells, thereby improving the cough and mucus symptoms of chronic bronchitis.
Building upon the positive results of previous studies, the RheSolve Trial is a double-blind, randomized, sham-controlled study in COPD patients with moderate to severe chronic bronchitis. The study will randomize 270 subjects in a 2:1 ratio at up to 40 US centers and up to 10 international centers.
The trial will assess the safety and efficacy of the RheOx System when used to treat the symptoms of chronic bronchitis. The primary efficacy endpoint is the change from baseline to 6 months in the COPD Assessment Test (CAT) score.
“Chronic bronchitis is a debilitating disease which results in symptoms that impact the quality of life which commonly persist following guideline-based medical treatment. Our collaboration with the COPD Foundation to bring forward the CAT score as a meaningful patient-reported measurement helped enable a study design centered on an outcome reflecting the patient’s experience,” said Frank Sciurba, MD, Professor of Medicine in the Division of Pulmonary and Critical Care Medicine at the University of Pittsburgh, and Co-Principal Investigator. “Prior feasibility study results suggest that Bronchial Rheoplasty using RheOx is well-tolerated, reduces symptoms, and may significantly improve quality of life for patients. We look forward to studying this therapy in a larger, blinded, randomized trial.”
“With the approval of this rigorously designed trial, we are ready to commence enrollment at major medical centers across the United States,” said Jonathan Waldstreicher, MD, CEO of Gala Therapeutics. “This is a significant milestone towards bringing a breakthrough therapy to millions of chronic bronchitis patients in the US.”