With the COVID-19 pandemic ongoing, Thirona starts a new collaboration to further improve and accelerate the detection of the virus. SmartCAD | COVID-19 is a software product that combines Thirona’s CAD4COVID-CT algorithm with the popular COVID-19 reporting template developed by digital health company Smart Reporting.
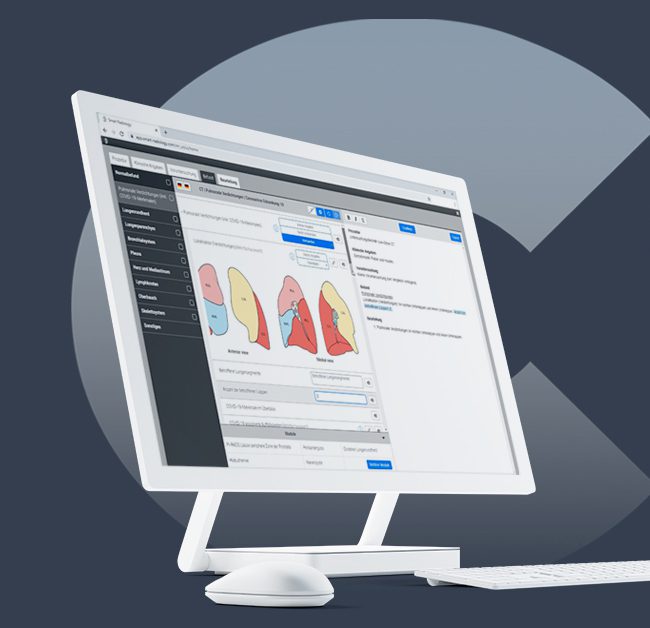
The innovative new system enables direct analysis of chest CT images with CAD4COVID-CT and transfers the results straight into a structured report. The output includes a COVID-19 severity score on lung and lobar level, the percentage of abnormalities detected for each lobe and the lungs overall, and the percentage of lobar emphysema. This way, SmartCAD | COVID-19 combines radiologist’s findings with the data of the CAD4COVID-CT AI algorithm in an insightful way. It is fast, cloud-based, and provided free-of-charge in support of the fight against the pandemic.
Support in diagnosis, evaluation, and documentation: Smart Reporting offers templates for radiologists to record their insights on a patient’s clinical status. Thirona’s CAD4COVID-CT algorithm results are now integrated in the COVID-19 template to support in the diagnosis of suspected cases. CAD4COVID-CT software uses a single inspiratory chest CT scan in the form of a DICOM image and results can be sent straight into the Smart Reporting template. SmartCAD | COVID-19 provides a structured approach to report abnormal findings in chest CT, highlighting findings that may be associated with COVID-19 affecting the lungs. The report includes not only findings by the radiologist but also pre-filled insights offered by the CAD4COVID-CT AI algorithm. It can be used as support in diagnosis, evaluation, and documentation of pulmonary tissue images from CT thoracic datasets.
Used by radiologists worldwide: Dr. Thomas Huber, Senior Clinical Product Manager at Smart Reporting, about SmartCAD | COVID-19: “Thanks to our long-standing cooperation with Thirona we could quickly react to a pandemic like the one we are facing now and integrate the CAD4COVID-CT software into our reporting template. It provides a semi-automatic workflow that assists physicians in diagnosing the virus. Our free structured templates are used by radiologists worldwide, making this new addition an easy next step.” Dr. Eva van Rikxoort, Managing Director of Thirona, adds: “CAD4COVID-CT can not only detect if someone is infected with the virus but also quantify how much and which parts of the lungs are affected. And because AI is available 24/7, patients always get the same high-quality analysis of their scan. It is a new tool in helping to find active cases, so healthcare professionals can react accordingly. Through the collaboration with Smart Reporting, CAD4COVID-CT is instantly accessible to 10,000 radiologists that are already registered on the platform.”