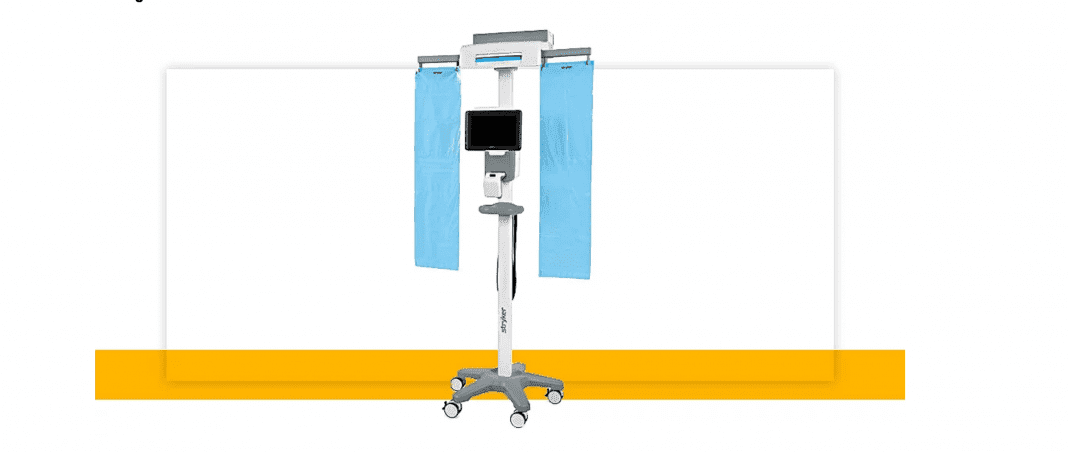
SurgiCount+ Safety-Sponge System, is Stryker’s next generation surgical sponge counting technology.
SurgiCount+ is Stryker’s most intelligent surgical sponge counting technology and allows healthcare providers to count and scan sponges and connect to hospital electronic medical records (EMR). Built on Stryker’s existing technology, it is designed to assist with sponge management, a leading safety concern in the operating room (OR) due to inadequate tools, manual processes and inconsistent practices.
One out of every 11 cases has a missing sponge,1 resulting in nurses spending almost 10 minutes searching.2 Current data show sponges are the number one retained surgical item3 – occurring 11 times a day in the U.S. alone.1 This negatively impacts patients and causes millions of dollars in costs for hospitals each year.
“Stryker is collaborating with surgical teams across the country to create safer ORs and equip healthcare providers with the tools needed to avoid retained surgical sponges,” said Mike Carlin, Vice President and General Manager of Stryker’s Surgical Technologies business. “We want healthcare providers to know they can count on us to make zero retained surgical sponges* the expectation and reality and provide certainty when they need it most.”
SurgiCount+ advances Stryker’s mission of making healthcare better by simplifying workflow and streamlining processes, resulting in less time spent searching for missing sponges. To date, medical staff have trusted SurgiCount in over 16 million procedures with zero retained sponges*. Features of SurgiCount+ include:
- UHF-RFID tagged sponges enabling unique identification, eliminating false-correct, duplicate or unknown counts
- A wireless reader for scanning, counting and finding sponges in the operating room
- Intuitive, user-friendly tablet interface
- A workstation stand equipped with sponge hanging bags and sterile reader covers situated on wheels for easy maneuverability and minimal floor space
- A digital platform enabling EMR connectivity and documented proof
1 Makary, M et al. “Medical error—the third leading cause of death in the US.” The BMJ (May 2016). Web. Sept. 2016.
2 Double-blinded survey of 154 Operating Room Nurses from 154 different facilities across the United States. Data on file internally. Conducted August 2020.
3 The Joint Commission. “Preventing unintended retained foreign objects.” Sentinel Event Alert. Issue 51. Oct. 2013.
* When used and implemented correctly