What To Know
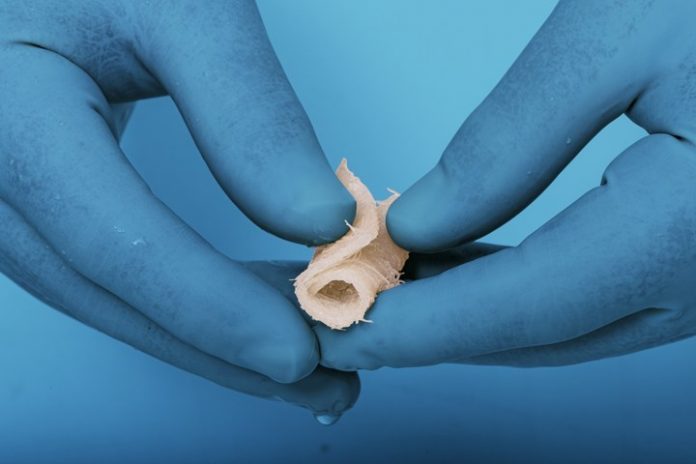
- The unique and versatile DBF Fiber Wrap is provided as a lyophilized sheet, comprised of 100% demineralized cortical bone fibers, that may be rehydrated with saline or the patient’s blood or bone marrow, creating a strong yet flexible sheet that can be manipulated by the surgeon.
- TheraCell’s patented TheraFuze DBF processes have been developed to produce uniform fiber geometry, size and texture, and to preserve the natural collagen structure of the allograft, providing surgeons with a 100% natural allograft with a cell-friendly nanotopography.
TheraCell Inc. announced that it has completed the first surgical case using its TheraFuze DBF Fiber Wrap. This is the third new product in the company’s portfolio of procedure-specific fiber graft solutions to be implanted in just the past several weeks.
The unique and versatile DBF Fiber Wrap is provided as a lyophilized sheet, comprised of 100% demineralized cortical bone fibers, that may be rehydrated with saline or the patient’s blood or bone marrow, creating a strong yet flexible sheet that can be manipulated by the surgeon.
The first surgical case using this novel procedure-specific product was performed by Dr. Neel Anand, MD, Mch. Orth. (Liverpool), FAAOS, Professor of Orthopaedic Surgery and Director, Spine Trauma, Cedars Sinai Medical Center, and Chairman of Orthopaedics and Spine, Minimally invasive Spine Surgery, DOCS Surgical Hospital, Beverly Hills. The case was a revision procedure involving posterolateral fusion at L5-S1 and T11-12. Dr. Anand added autograft and TheraFuze® FiberForm™ to the Fiber Wraps, rolled them to contain the graft material, and placed them in the posterolateral gutters.
“I was very impressed by how easily the Fiber Wraps rehydrated and could be rolled up like a burrito,” said Dr. Anand. “It was remarkable how well the sheet held the additional graft materials and I came away with a strong sense of confidence that the graft would stay in place.”
The TheraFuze DBF Fiber Wraps are made from cortical demineralized bone fibers and processed using TheraCell’s proprietary Bone Textile™ technology. TheraCell’s patented TheraFuze DBF processes have been developed to produce uniform fiber geometry, size and texture, and to preserve the natural collagen structure of the allograft, providing surgeons with a 100% natural allograft with a cell-friendly nanotopography. TheraCell’s FiberLok™ technology allows the Fiber Wrap products to maintain their form and integrity after rehydration permitting adequate time for manipulation and implantation.
The TheraFuze DBF Fiber Wraps are part of TheraCell’s comprehensive portfolio of TheraFuze DBF procedure-specific graft solutions, currently available in the United States directly through TheraCell. “We are pleased to have achieved our 2020 product launch objectives for the TheraFuze DBF product line, and we look forward to commercializing a few additional products in the first half of 2021,” said Andy Carter, PhD, TheraCell’s Chief Technology Officer.