What To Know
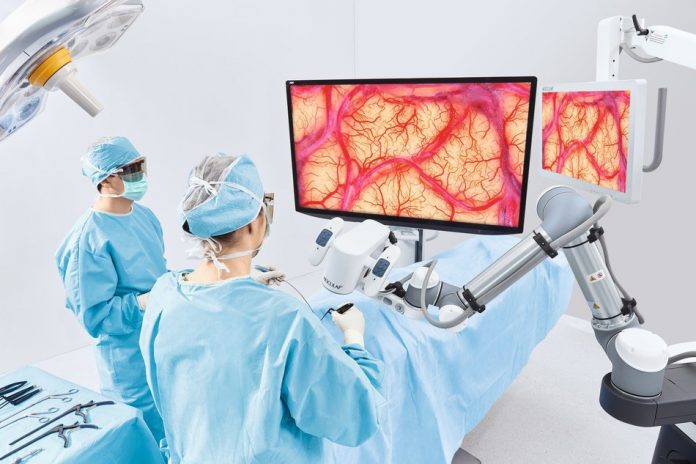
- The innovative Image Modes feature of the Aesculap Aeos Robotic Digital Microscope delivers augmentation capabilities without the need for an overlay, and the platform also includes 3D details with outstanding depth-of-field, fluorescence backlighting capabilities and a 10x optical zoom that delivers full resolution regardless of magnification level.
- The Aesculap Aeos Robotic Digital Microscope is powered by TDS, the California-based leader in digital microscopy with a nearly 20-year legacy of creating new technologies that are designed to meet the precision needs of surgeons today.
True Digital Surgery (TDS) and Aesculap, Inc. today announced that the Aesculap Aeos™ Robotic Digital Microscope is now available in the United States.
This innovative platform enables surgeons to execute precision movements in certain procedures, including neurosurgery, spine, and ear, nose and throat operations, through a multi-axis robotic arm. The Aesculap Aeos Robotic Digital Microscope adds state-of-the art software capabilities, providing a 3D digital visualization display, customizable Image Modes functionality, and fluorescence backlighting to enhance the precision needs that surgeons require for complex surgical procedures.
In the midst of the challenges of the COVID-19 pandemic, TDS and Aesculap, Inc. are launching the innovative Aesculap Aeos Robotic Digital Microscope to also offer potential improvements to safety and efficiency during surgeries, as the heads-up display can be viewed simultaneously by all healthcare workers in the operating room while wearing PPE.
The innovative Image Modes feature of the Aesculap Aeos Robotic Digital Microscope delivers augmentation capabilities without the need for an overlay, and the platform also includes 3D details with outstanding depth-of-field, fluorescence backlighting capabilities and a 10x optical zoom that delivers full resolution regardless of magnification level. As an exclusively digital microscope platform, it can be customized with simple software updates.
“We are proud to bring the fully-digital Aesculap Aeos Robotic Digital Microscope to market, with breakthrough technology that we like to say can ‘see beyond the tissue’ and provide a solution to the limitations of conventional surgical microscopes, especially during this challenging time for the global medical community,” said Aidan Foley, Chairman and CEO, True Digital Surgery. “This platform was developed with True Digital Surgery’s unmatched experience in 3D visualization and vivid imagery, coupled with the invaluable insights and global sales and support capabilities gained from our strategic partnership with Aesculap.”
Approximately four out of five neurosurgeons report pain after a day of surgery, in part owing to conventional microscope technology that requires contorting their bodies in unergonomic postures for many hours. This often leads to debilitating pain which has been found to affect the performance of surgeons.1
The Aesculap Aeos Robotic Digital Microscope is powered by TDS, the California-based leader in digital microscopy with a nearly 20-year legacy of creating new technologies that are designed to meet the precision needs of surgeons today. The company’s technology is utilized across multiple disciplines, including ophthalmology, neurosurgery, ENT and spinal surgeries, by prominent medical device partners under a variety of brands and private labels. TDS closed a Series B funding round raising $16 million in December 2019.
“We are thrilled to collaborate with TDS and offer this unparalleled technology to U.S. surgeons and medical systems,” said Chuck DiNardo, President of Aesculap, Inc. “The Aesculap Aeos Robotic Digital Microscope is a strategically important addition to our U.S. portfolio, which provides healthcare providers with industry-leading advancements developed through a responsive approach that is focused on innovation, efficiency and sustainability.”
To develop the Aesculap Aeos Robotic Digital Microscope, TDS partnered with Aesculap, Inc., a subsidiary of B. Braun, a global company with an over 150-year legacy in developing, manufacturing and marketing innovative medical products and services to the healthcare industry. In addition to the U.S., the Aesculap Aeos Robotic Digital Microscope is also approved for use and available in the European Union and further markets.
“Advances in healthcare are based on a very close cooperation with our customers, and require a willingness to face change with innovative solutions, shaping the future of medicine,” said Andreas Knapp, Vice President Global Marketing and R&D Neurosurgery and Power Systems, Aesculap AG. “Together with True Digital Surgery, we have developed such an innovative solution with the new Aesculap Aeos. Through this strategic partnership, we are pleased to market this fully digital surgical microscope platform globally, fulfilling the most important requirements of our surgeons with regards to superior visualization capabilities, robotic-assisted positioning and improved ergonomics.”
Reference
Davis WT, Fletcher SA, Guillamondegui OD. Musculoskeletal occupational injury among surgeons: effects for patients, providers, and institutions. J Surg Res. 2014;189(2):207-12 e6