What To Know
- 2 The modules are designed to ensure that the right clinical decision is made at the right time, no matter where the patient resides in the healthcare system, to ensure quick and appropriate care.
- Ai is the leader in AI-powered care coordination and has accelerated the time-to-notification of the treatment team by 73 percent and time-to-treatment by 24 percent.
Viz.ai – the world leader in AI-driven intelligent care coordination, announced the U.S. commercial launch of its AI-powered modules for pulmonary embolism and aortic disease. Debuting at the 48th annual VEITHsymposium November 16 – 20 in Orlando, Fla., the new modules allow for faster clinical decision making and improved care coordination for patients suffering from these two life-threatening conditions.
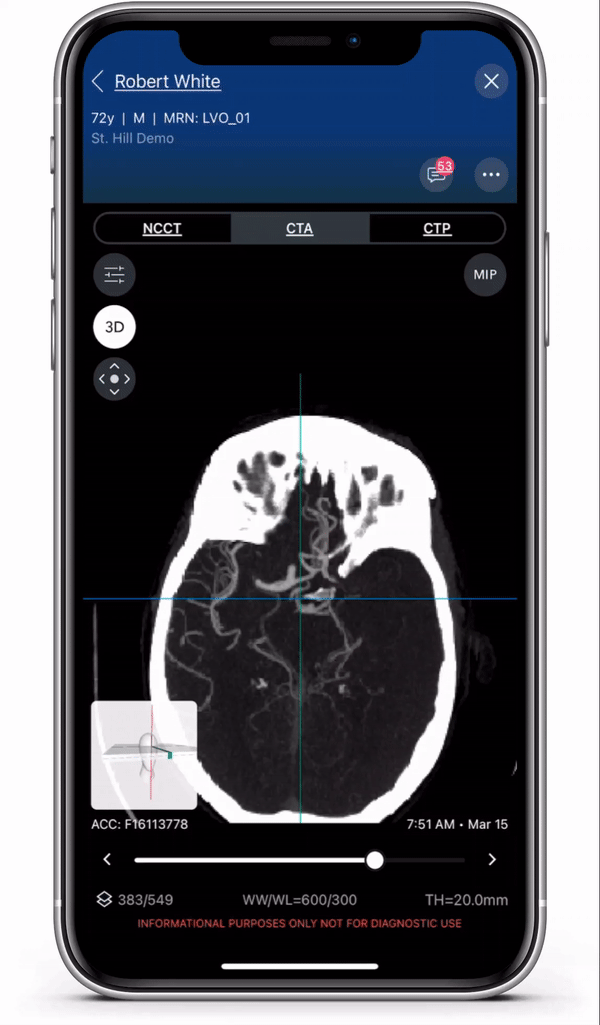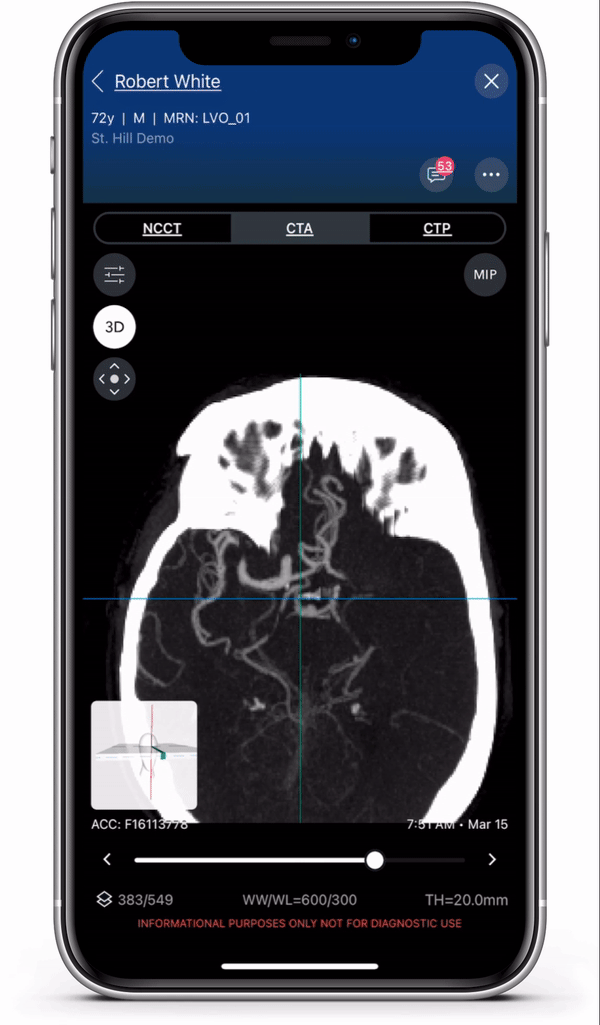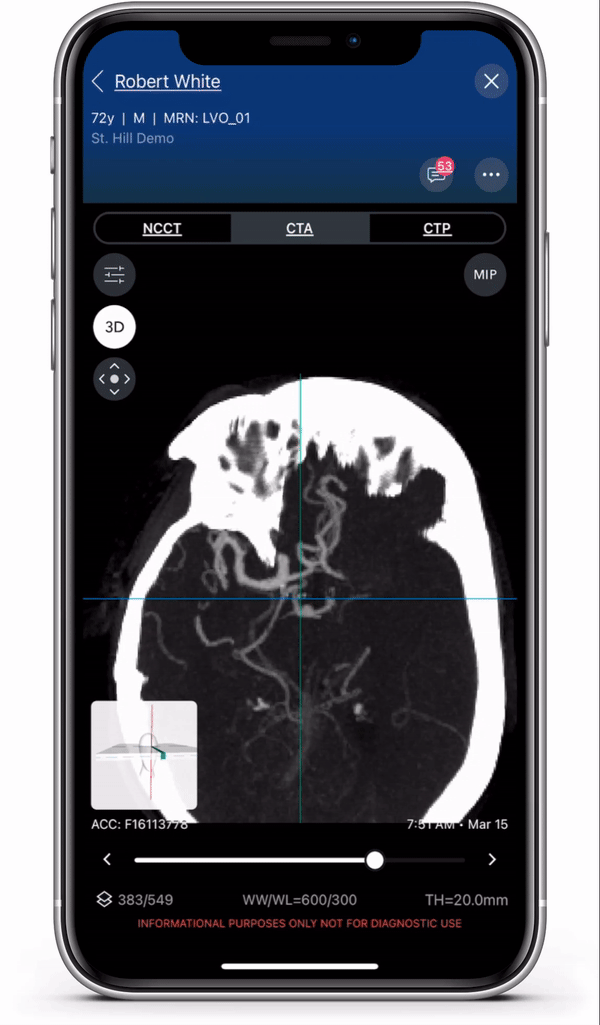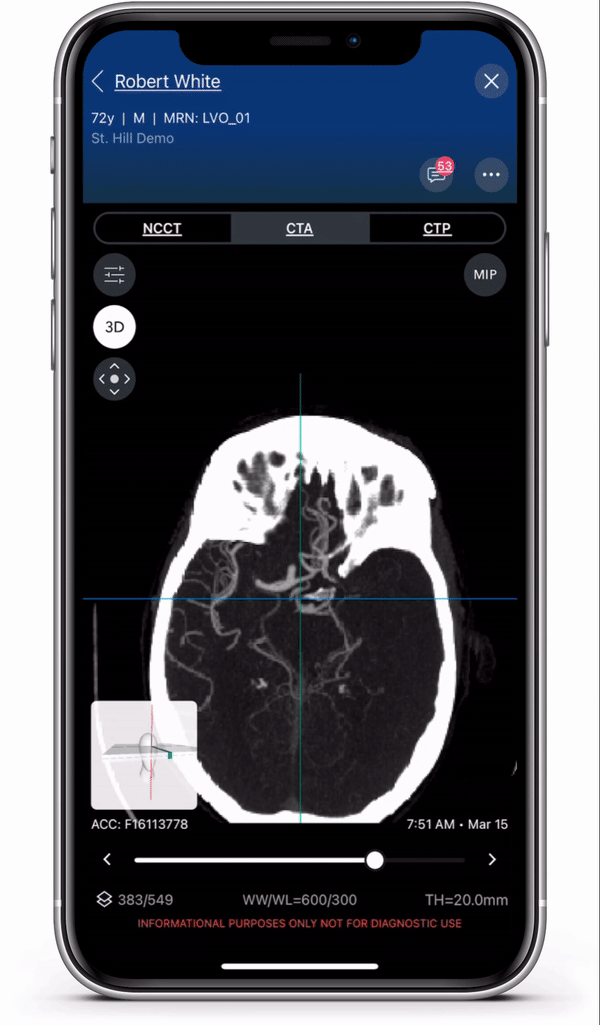
Diagnosis and care coordination of patients suffering from PE and aortic disease can be challenging, with the average arrival-to-treatment times lasting more than 8 hours for these emergent conditions.1 Viz.ai uses deep learning to identify suspected aortic dissection and pulmonary embolism disease in under two minutes.2 The modules are designed to ensure that the right clinical decision is made at the right time, no matter where the patient resides in the healthcare system, to ensure quick and appropriate care. Viz.ai is the only software company to offer an AI-powered care coordination solution for stroke, aortic disease, and pulmonary embolism.
The new modules leverage advanced deep learning to communicate time-sensitive information, including advanced imaging studies and real time clinical information, to specialists who can more quickly and easily make treatment decisions for the patient. Users can access all aortic and pulmonary imaging from the cloud, enabling care coordination regardless of geography.
“This technology has changed the way we triage and treat stroke patients, dramatically improving their care,” said Dr. Richard Saxon, interventional radiologist at Tri-City and Palomar Health, San Marcos, Calif. “In a similar fashion, I believe the Viz.ai PE and Aortic modules will enhance image interpretation with AI, dramatically improve the quality and speed of mobile image viewing, and improve caregiver communication. This is truly the care coordination of the future.”
Viz.ai is the leader in AI-powered care coordination and has accelerated the time-to-notification of the treatment team by 73 percent and time-to-treatment by 24 percent.3 The Viz Platform is now utilized in over 850 hospitals across the U.S. and Europe and touches almost two patients every minute.
“The launch of these two new modules brings the power of the Viz Platform to a vast new faction of medical professionals and patients battling life-threatening conditions,” said Dr. Chris Mansi, Viz.ai CEO and co-founder. “This is a significant milestone in our mission to fundamentally improve how healthcare is delivered in the world.”
Viz.ai will be showcasing the new AI-powered PE and aortic disease modules at VEITHsymposium Booth # 317.