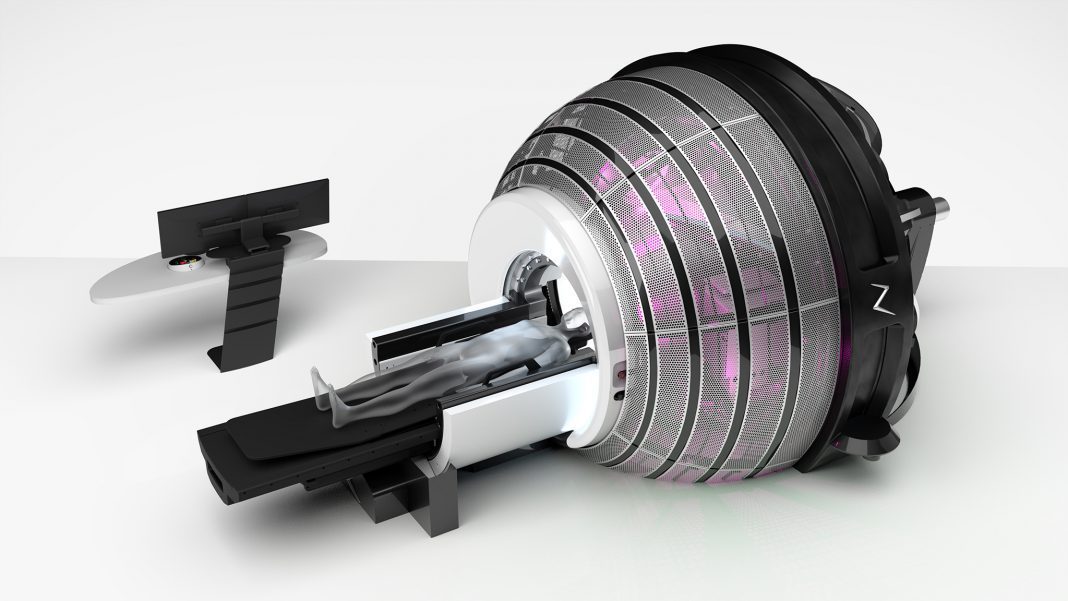
ZAP Surgical Systems, Inc. today announced an agreement to bring its advanced ZAP-X® Gyroscopic Radiosurgery® platform to the Centre de Cancérologie de la Porte de Saint-Cloud (CCPSC) in Boulogne, France. As part of the renown American Hospital of Paris, CCPSC will be the first in France to offer the latest advance in radiosurgical brain tumor treatments. First patient treatments with ZAP-X at CCPSC are estimated to begin the Fall of 2022.
Stereotactic radiosurgery (SRS) is a well-established procedure for the non-invasive treatment of many primary and metastatic brain tumors, as well cranial functional and vascular disorders including trigeminal neuralgia and arteriovenous malformations. Often considered an alternative to costly and invasive surgical procedures, SRS is a non-invasive outpatient procedure that often provides superior outcomes, yet requires no surgical incision, and little to no patient recovery period.
“A significant percentage of cancer patients develop brain metastases during the course of their disease,” said Eric Duret, General Manager of CCSPC. “As this population continues to grow with improving cancer survival, having the most advanced radiosurgery option available to our patients was imperative.”
The ZAP-X system uses unique gyroscopic mobility to direct radiosurgical beams from hundreds of angles to precisely concentrate radiation on the tumor target. This pioneering approach supports the clinical objective of protecting healthy brain tissue and patient cognitive function, and when needed, enable future potential SRS re-treatments without the unnecessary risks associated with other radiation delivery techniques.
ZAP-X® Gyroscopic Radiosurgery® platform is additionally recognized for being the first and only vault-free and cobalt-free dedicated SRS delivery system, thereby eliminating the costs to build expensive shielded radiation treatment rooms, and removing the need to maintain, secure and regularly replace live radioactive isotopes.