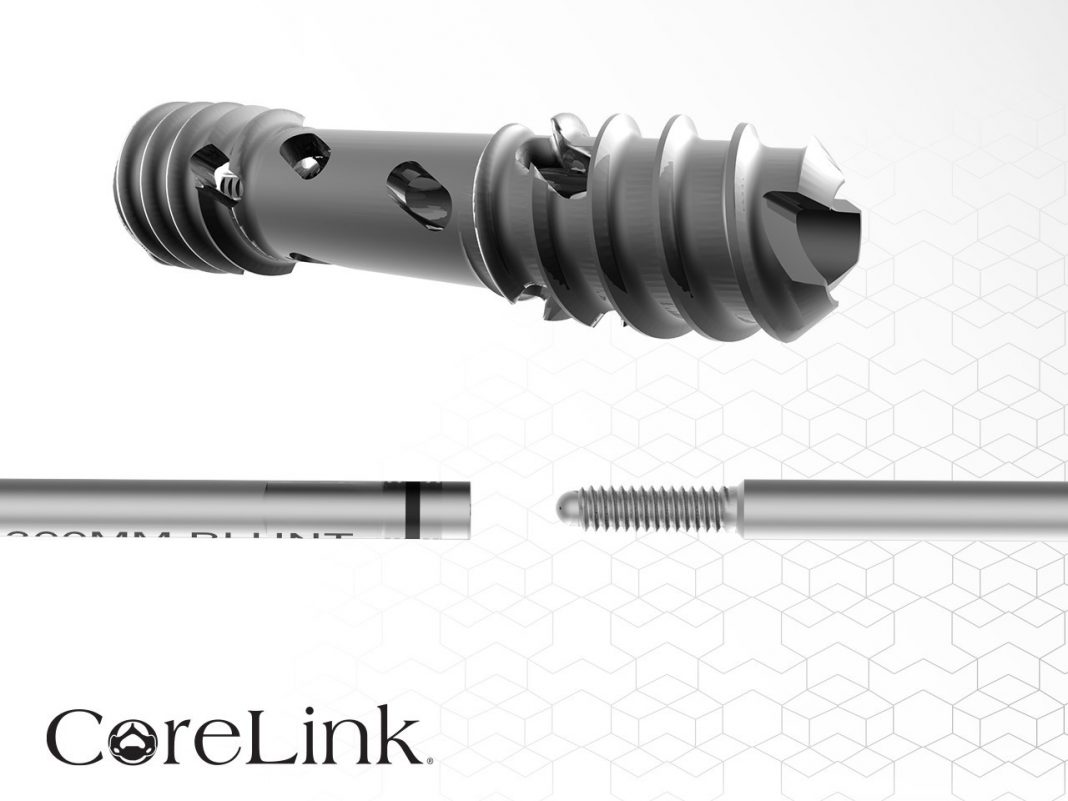
CoreLink, LLC, a leading designer and manufacturer of spinal implant systems, today announced the implantation of over 5,000 Entasis® Lateral SI Joint Fusion System compression screws to fuse the sacroiliac joint. View more.
CoreLink also announced the company has been awarded patent number 11,052,229 by the United States Patent and Trademark Office (USPTO). The patent, titled, “Devices and methods for guidewire extension in spinal surgery,” expands the company’s intellectual property portfolio. These unique stackable guide wires are designed to give surgeons streamlined instruments and constant proximal control during every step of these lateral SI joint fusion procedures.
With the spine market expected to increase 5 percent this year1 and sacroiliac joint fusion growing up to 14.7 percent2, SI joint fusion continues to be an exciting new area of spine surgery. Lower back pain is one of the most common reasons for a physician visit and those experiencing pain attributable to the SI joint range from 15 to 30 percent3.
Entasis is designed for minimally invasive SI joint fusion procedures. The system provides an array of joint stabilizing compression screw options in three diameters and nine length options, enabling surgeons to precisely fit varying patient anatomies. The screws feature circumferential fenestrations that self-harvest bone graft while compressing the joint. Dual leads threads ease insertion and easy-out threaded removal tools are available, if needed. These innovative features and stackable guide wire have led to the Entasis system being recognized as a top 10 sacroiliac joint fusion system by SPINEMarketGroup. A full suite of resources to assist in surgeon training, patient education, and patient workup is also available.
“CoreLink is proud to be a leader in the SI fusion market as a result of our strong product offering and vast training resources,” said Jay Bartling, CEO, CoreLink. “Every step of our process, from design to manufacturing, keeps surgeon ease of use and patient outcomes top of mind. This is evident in our SI joint fusion systems with patented technology, unique compression screw design, and comprehensive offerings.”
CoreLink will be exhibiting at the North American Spine Society’s annual meeting in Boston, September 29 through October 2, booth #3221, where a full display of surgical systems will be featured, including Entasis.
References
|
1. |
The Spine Market Group. (2021). What is the Spine Market First Estimate for 2021. Retrieved from https://thespinemarketgroup.com/which-is-the-spine-market-first-estimate-for-2021. |
|
2. |
360 Research Reports. (2020). Global MIS Sacroiliac Joint Fusion Sales Market Report 2020. Retrieved from https://www.360researchreports.com/global-mis-sacroiliac-joint-fusion-sales-market-16695557. |
|
3. |
Martin, C. T., Haase, L., Lender, P. A., & Polly, D. W. (2020). Minimally Invasive Sacroiliac Joint Fusion: The Current Evidence. International journal of spine surgery, 14(Suppl 1), 20–29. https://doi.org/10.14444/6072 |