These awards are given to the top-performing Medline distribution centers in the United States across its four tiers of size, as determined by the sales volume distributed out of each facility
Medical Device Industry News
Latest Developments, Breakthroughs, and Future Outlook
Medical Device News Magazine takes pride in delivering high-quality, in-depth news. Our content goes beyond the surface, providing a comprehensive look at the trends, innovations, and breakthroughs that are shaping the future of medical technology.
In addition, we aim to offer a unique perspective on the rapidly evolving healthcare landscape. By following our news, you gain exclusive insights into industry developments, emerging technologies, and regulatory updates that impact medical devices worldwide. At the same time, you become part of a vibrant community of professionals, innovators, and thought leaders who are dedicated to advancing healthcare through cutting-edge solutions.
Furthermore, we encourage you to explore our diverse range of content, from the latest product announcements to in-depth analyses of industry trends. If our vision aligns with your objectives, we also offer opportunities for advertising and collaboration, allowing you to reach a highly engaged audience of medical technology professionals.
Latest News and Developments
Stryker launches SmartHospital Platform
"We are dedicated to partnering with our customers on their digital journeys to help elevate care delivery," said Scott Sagehorn, VP/GM of Smart Care at Stryker. "The SmartHospital Platform is designed to evolve alongside health systems so teams can work more efficiently and stay focused on patient-centered care."
1st Patient Treated with ResQFoam for Life-Threatening Abdominal Bleeding | Reports Arsenal Medical
FDA Breakthrough-designated device under evaluation for treatment of life-threatening abdominal bleeding at leading U.S. trauma centers shows promising results in first patient
Clinilabs Establishes EU Headquarters in Basel, Strengthening European Clinical Operations Under New Regional Leadership
The company also announced the appointment of Dr. Anne-Marie Nagy as executive vice president and head of Clinilabs Europe.
CharmHealth Advances Its AI Strategy With MCP Server
By embedding AI as core infrastructure rather than an add-on, CharmHealth is advancing a more practical, governed approach to AI adoption with the EHR
Primera Selects Cenevo’s Labguru to Transition from Paper to Digitalized Workflow
“Beyond our specialized CRO solution set, our client-facing team has extensive real-world CRO experience,” said Keith Hale, CEO of Cenevo. “Our tailored solution and industry experience will help guide Primera through the steps they need to fully embrace automation and digitalization in their highly specialized environment.”
Clinical Trials
MBX Biosciences Announces Successful End-of-Phase 2 FDA Meeting and Provides Phase 3 Development Plan for Once-Weekly Canvuparatide for Hypoparathyroidism
Following End-of-Phase 2 meeting, MBX remains on track to initiate Phase 3 in Q3 2026
Medicus Pharma Provides Interpretation of Positive Phase 2 SkinJect™ Dataset
The Company Reported 73% Clinical Clearance in the 200-µg cohort at Day 57
Medicus Pharma reports positive Phase 2 SKNJCT-003 Topline Data observing 73% Clinical Clearance and 40% Histological Clearance at day 57
The company is expected to finalize the Clinical Study Report (CSR) in Q2 2026 to support planned end of Phase 2 (EOP2) meeting with the FDA
Biotechnology News
Evinova Announces Strategic Collaborations with Astellas, AstraZeneca and Bristol Myers Squibb Advancing Its AI-Native Platform to Accelerate Global Clinical Development
Each partner company has opted in to share operational data with Evinova, enabling the platform to provide benchmarks and smarter recommendations intended to further accelerate clinical trials and improve patient outcomes
Cenevo Advances Toward Agentic Labs with Two New AI Agents
The new agents are the AI Protocol Conversion agent and the AI Automation agent.
Comprehensive Insights into Medical Device & Biotechnology Company Mergers, Acquisitions & Funding
Abram Scientific Secures $11.75M Series A Funding Led by Octapharma AG
Financing will accelerate the development of proprietary portable diagnostic platform, CoagCareTM, to enhance patient care for bleeding disorders & critical care medicine
ProSomnus Secures $38 Million Strategic Investment from Catalio Capital Management to Scale Smart Sleep Medicine™
Strategic alliance focuses on scaling data-integrated sleep solutions to meet surging clinical and consumer demand

Simpson Interventions Announces Initial Series C Close and Rebrands as Elumn8 Medical
Series C financing supports continued clinical development of the Acolyte™ Image-Guided coronary chronic total occlusion platform, an FDA Breakthrough Device

Johnson & Johnson Boosts U.S. Presence with $1 Billion+ Investment in Next‑Gen Cell Therapy Facility
Advanced manufacturing site will utilize cutting‑edge cell therapy technologies to help deliver the company’s portfolio of transformational medicines
Industry Expert Bylines

The Hidden Economic Cost of Falling Vaccination Rates | By David A. Dodd – CEO of GeoVax
Even small declines in measles vaccination coverage costs the United States billions, revealing why prevention infrastructure matters more than emergency response

How an Intelligent QR Architecture and GS1 Digital Links Can Improve Medical Device Recall Workflows
By Ravi Pratap Maddimsetty, co-founder and CTO of Uniqode, responsible for all technology strategy, product innovation and engineering execution

HTM’s next frontier: AI, intelligent operations, and tech-driven resilience in 2026 | By Neil de Crescenzo, Chief Executive Officer at TRIMEDX
In this ever-changing healthcare landscape, prioritizing data-driven decision-making and proactively adopting advanced technologies will position health systems to achieve lasting success in 2026 and the years ahead
FDA Medical Device Updates
Market Reports
Airiver Medical Granted FDA’s Breakthrough Device Designation for Pulmonary Drug Coated Balloon to Treat Central Airway Stenosis; First Patient Treated in Clinical Trial
Designation may provide U.S. patients faster access to groundbreaking technology
Expanded FDA 510(k) Clearance Supercharges Bayer’s MEDRAD® MRXperion System for Broader MR Deployment
Clearance reinforces Bayer’s continued commitment to dependable, integrated radiology solutions
Executives on the Move

John P Landino Appointed Chief Development Officer at Healogics
He brings nearly 20 years of experience in business development, marked by a proven ability to forge strategic partnerships with hospital executives and healthcare leaders, supporting rapid and sustainable organizational growth

Michael Clay Named COO at Caidya
To drive operational excellence and advance multi-regional clinical development
First Ascent Biomedical Opens FPM Cancer Lab in Miami; Welcomes World-Class Clinical Leadership
Miami lab is now accepting patient and physician referrals. Eligible Florida residents and colorectal cancer patients nationwide may qualify for limited sponsored programs covering the cost of testing
Advancements In Imaging
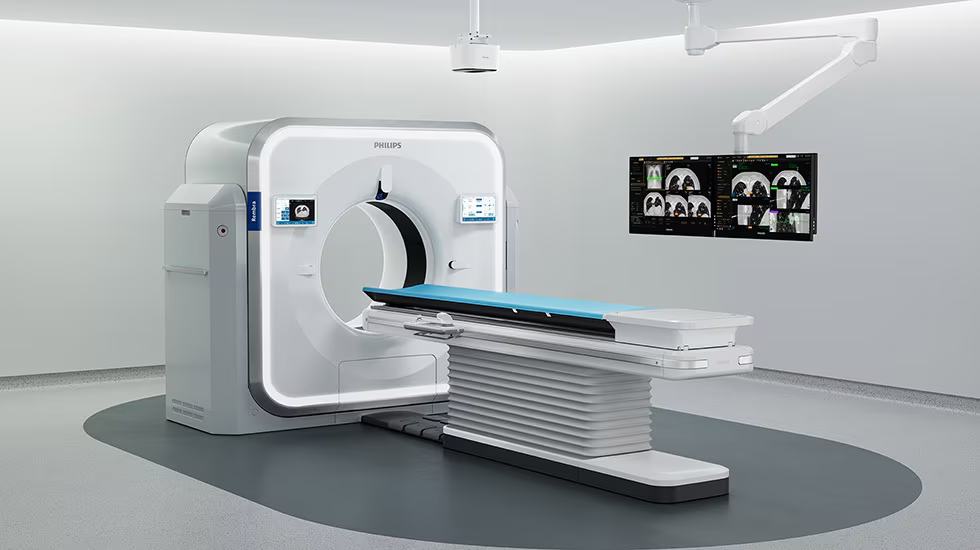
Philips unveils Rembra CT at ECR 2026, setting a new benchmark for speed and patient access designed to support diagnostic confidence for acute and high-demand imaging environments
CE Marked and 510(k) pending, Rembra’s advanced image reconstruction technology delivers up to 106 images per second [1] and a high throughput of up to 270 patients per day to support faster diagnosis by making scans available in near-real time
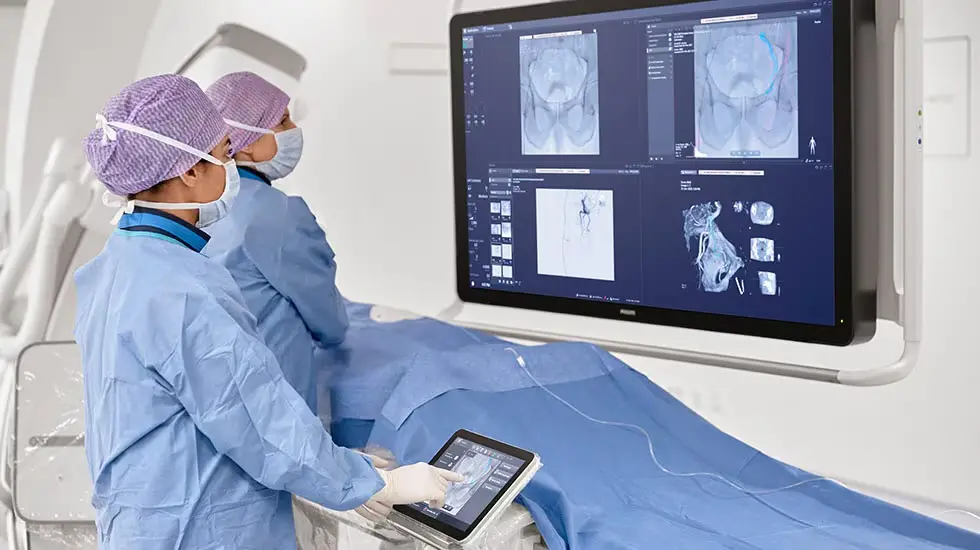
PreciseOnco research consortium awarded EUR 14.9 million IHI grant to drive breakthroughs in precision cancer treatment
Co-funded by the EU’s Innovative Health Initiative (IHI), the Philips-coordinated research consortium will combine advanced medical imaging, robotic guidance technologies, and AI to standardize and enhance the precision of minimally invasive cancer treatments
New Peer-Reviewed Study Validates AISAP’s AI Ability to Detect Major Heart Disease from a Single Ultrasound View
Retrospective Analysis of Over 120,000 Echocardiographic Studies Provides Clinical Evidence of Diagnostic Accuracy for Heart Failure and Valvular Disease
Hospitals In the News
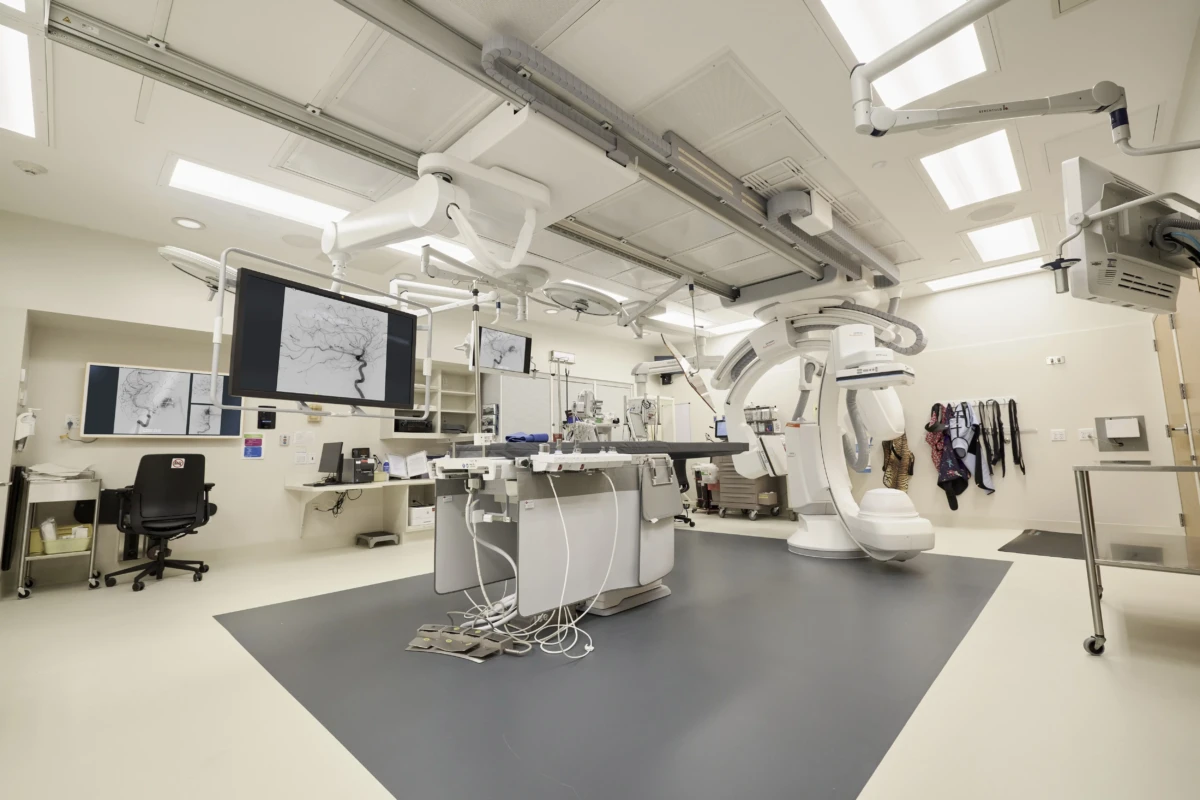
Children’s Minnesota Unveils Region’s First Dedicated Pediatric Neurointerventional Angiography Suite to Treat Stroke Patients
The new suite brings advanced treatment options to pediatric patients with various types of strokes, including blood vessel malformations causing bleeding in the brain, as well as blood clots

Good Samaritan Medical Center Successfully Performs Focal Cryoablation for Prostate Cancer Using Advanced KOELIS Trinity® Fusion and Varian Cryoablation Technology
Good Sam and Dr. Jue are the only hospital and surgeon from Orlando through Miami to perform the surgery

Kauvery Hospital, Alwarpet Performs One of the World’s First TAVR-in-TAVR-in SAVR with Bioprosthetic Valve Fracture in 78-Year-Old
The advanced transcatheter intervention was undertaken to treat a repeatedly failing heart valve in a patient with a long and challenging history of aortic valve disease
Non-Profit News
Minimally Invasive Procedure Effectively Treats Small Kidney Cancers
The research focused on patients with stage T1a renal cell carcinoma, a cancer that is increasingly found incidentally on CT scans performed for other reasons, such as imaging of the prostate or ovaries
Hope For The Warriors Awarded $3 Million Grant from Lilly Endowment Inc.
$3 million investment strengthens foundation for the next generation of military family support
ACC Announces Inaugural Fellow for the Thad and Gerry Waites Rural Cardiovascular Research Fellowship
The new award provides up to $70,000 in funding to a Fellow-in-Training or an early-career cardiologist
Healthcare: Understanding the Business Side of the Industry and Its Implications
Revolutionizing Production Efficiency with a Carton Packaging Machine
The Magic of Automation: How Carton Packaging Machines Transform Production Lines
Unleashing Speed: The Impact of Carton Packaging on Production Rates
The Staffing Problem That Starts Before Anyone Quits
The staffing challenges facing healthcare will not disappear

How to solve the registered nurse shortage in 2026
This article explains steps to take to solve our 2026 registered nurse shortage

Modern Alternatives to Traditional Hospital Beds for Long-Term Support
Today the market offers a growing variety of modern alternatives that blend therapeutic benefit with home-friendly design
Healthcare Careers

Medical Assistant Career | What It Takes
Medical Assistant Career Can Be Rich and Rewarding A Medical Assistant career is one of the fastest and most practical ways to enter the healthcare
How to Choose the Best Medical Career for Your Future
Choosing the best medical career isn’t about finding the “perfect” specialty; it’s about finding the one that fits you
Innovative Careers in Healthcare Leadership
The future of healthcare leadership will be defined by those who embrace continuous learning, adaptability, and innovation
Nurses Corner

Nurse Burnout: A Strategic Threat Hospitals Can’t Afford to Ignore
Burnout isn’t just “being tired.” It’s a chronic state of emotional, mental, and physical exhaustion caused by prolonged stress.

Nurses | How To In Shape
Many nurses manage to stay in great shape, both physically and mentally—despite the challenges

Best Clothing for Nurses: Comfort, Function, and Style
Choosing the right attire can significantly impact a nurse’s performance, safety, and overall well-being. Here’s a detailed guide to the best clothing for nurses