CVRx, Inc. is a developer of the world’s first FDA-approved neuromodulation device to treat the symptoms of heart failure.
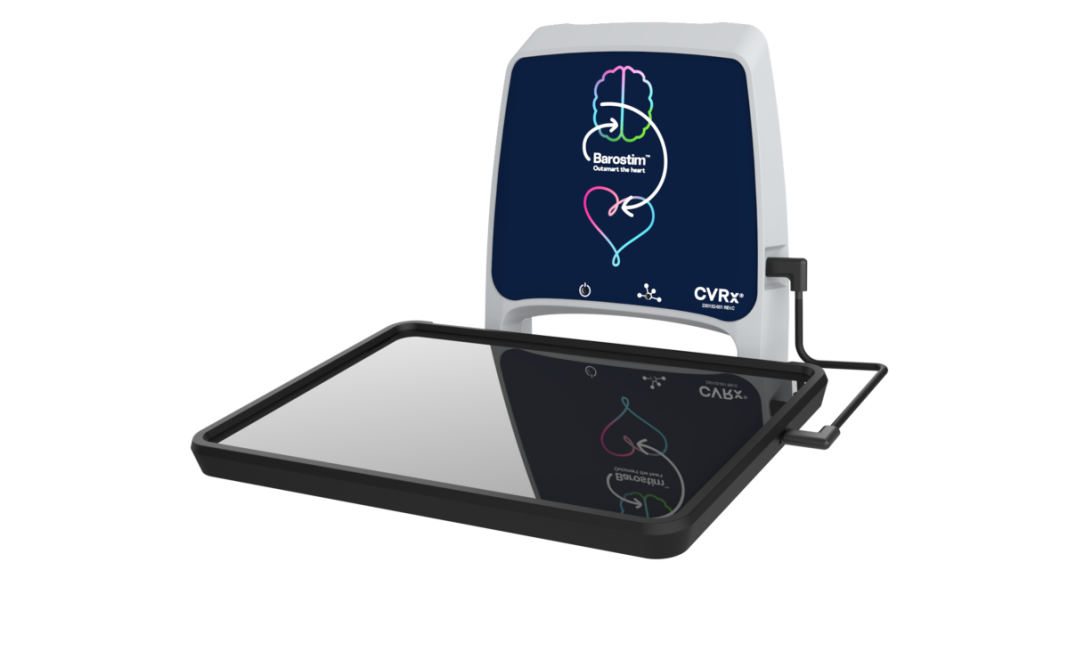
Today they announced the launch of its new Barostim Programmer, which was approved by the U.S. Food and Drug Administration (FDA) earlier this year.
The second-generation programmer has a modernized design, operates on an upgraded cellular network that enables remote view access, and includes an improved user interface that simplifies implantable pulse generator (IPG) programming. The new Barostim Programmer is compatible with all Barostim implantable systems.
“The new Barostim Programmer builds upon the earlier model and includes significant enhancements to both hardware and software,” said Nadim Yared, President and CEO of CVRx. “This upgrade allows CVRx to continue to provide superior customer support and reflects our commitment to innovating across all aspects of Barostim therapy.”
A complete rollout of the new Barostim Programmer is planned across the U.S. market throughout 2022.
CVRx is focused on the development and commercialization of the Barostim™ System, the first medical technology approved by FDA that uses neuromodulation to improve the symptoms of heart failure. Barostim is an implantable device that delivers electrical pulses to baroreceptors located in the wall of the carotid artery. Baroreceptors activate the body’s baroreflex, which in turn triggers an autonomic response to the heart. The therapy is designed to restore balance to the autonomic nervous system and thereby reduce the symptoms of heart failure. Barostim received the FDA Breakthrough Device designation and is FDA-approved for use in heart failure patients in the U.S. It has also received the CE Mark for heart failure and resistant hypertension in the European Economic Area.