December 7, 2020
RenalGuard Therapy better protects at-risk patients undergoing cardiac surgery with cardiopulmonary bypass (CPB) from Acute Kidney Injury (AKI) according to the KIDNEY clinical trial reports RenalGuard Solutions, a pioneering medical device company focused on protecting patients from AK.
Results of the 220 patient, randomized trial comparing automated diuresis technology to standard hydration were published in the European Journal of Cardio-Thoracic Surgery. The 220-patient study was conducted by investigators at the Heart and Lung Centre, New Cross Hospital in Wolverhampton, United Kingdom.
Millions of elective cardiac surgeries are performed each year worldwide, and AKI is the most common major surgical complication, reported in up to 30% of cases. In emergent cases, AKI rates can be twice as high, ranging from 50-70%. AKI complicates patient recovery, adds significant recovery time and is one of the strongest predictors of in-hospital and long-term mortality after surgery.
In the KIDNEY trial, patients at-risk for AKI were randomized into either a RenalGuard Therapy treatment group or a control group managed with standard-of-care hydration. Risk factors for AKI included a history of diabetes and or anemia; estimated Glomerular Filtration Rate (eGFR) < 60 ml/min/1.72m2; anticipated cardiac surgery time >120 minutes and Log EuroScore > 5. AKI was defined as a rise in serum creatinine greater than 50% in the days post-procedure.
The RenalGuard group’s post-operative AKI rates were significantly lower than the control group’s, 10% vs 20.9%. As well, the mean volumes of urine produced during surgery (2337 + 896 ml vs 766 + 557 ml) and within 24 hours post-op in ICU (3297 + 1298 ml vs 2053 + 802 ml) were significantly higher in the RenalGuard group. Median ICU stays were similar for the two groups.
The investigators led by Dr. Heyman Luckraz concluded that: “In patients at risk for AKI who had cardiac surgery with CPB, the RG system significantly reduced the incidence of AKI and can be used safely and reproducibly.”
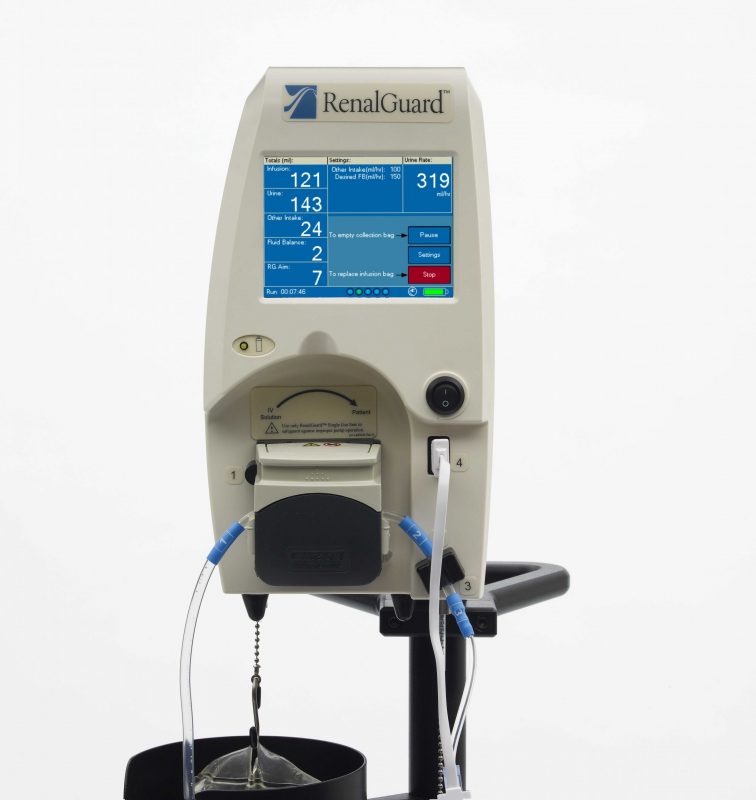
RenalGuard Therapy entails use of RenalGuard’s automated fluid management system with a physician-prescribed loop diuretic to induce high urine output rates, which protect against AKI. Used with more than 30,000 patients, the automated fluid management system is designed to measure urine output and replace it in real-time with an equal volume of sterile saline.
“We are very gratified by the results of this comprehensive study of RenalGuard in the cardiac surgery setting, which show this technology’s promise in this area as a means to protect a very high-risk population from cardiac surgery-associated AKI,” said RenalGuard Solutions President and Chief Executive Officer, Jim Dillon. “We look forward to further exploring the use of RenalGuard to reduce the incidence of AKI in this patient population.”