December 22, 2020
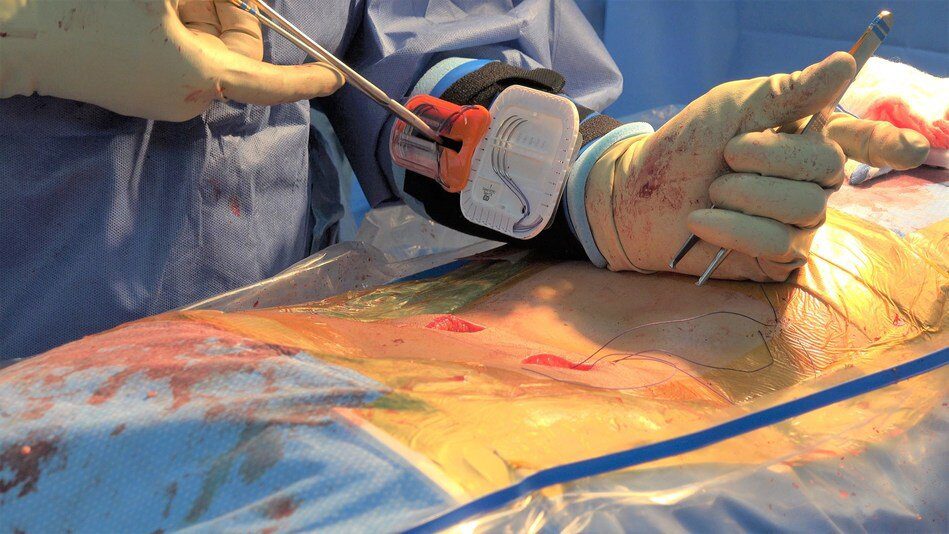
Sharp Fluidics reports the first prospective, case-controlled clinical study of an Engineered Sharps Injury Prevention (ESIP) device to prevent Needle Stick Injuries (NSI) in an operating room demonstrated that the use of Operative Armour®, a wearable needle safety and workflow efficiency device, reduces surgical needle passes and handling during surgical closure.
This study, “Efficacy of a Novel Intraoperative Engineered Sharps Injury Prevention Device: Pilot Usability and Efficacy Trial,” Jenny Hillary, M.D., et al. conducted at The Johns Hopkins University School of Medicine, was published in JMIR Perioperative Medicine.
In the 100-patient study, 50 procedures were performed using Operative Armour and 50 control procedures without. The procedures performed were in Plastics Surgery including abdominal surgery, breast reconstruction, and mastopexy/breast reduction or revisions. During these procedures, Operative Armour resulted in as many as half the needle adjustments by hand.
“In this study at Johns Hopkins it was found that Operative Armour functions effectively as an engineered Sharps Injury Prevention device by decreasing intraoperative needle passing and handling”, said Douglas Rimer, President of Sharp Fluidics. “The authors stated, ‘By minimizing sharps behaviors that drive needle stick injuries such as manipulation, handling, and passing of intraoperative sharps, Operative Armour demonstrates superiority over the current practice in the potential to significantly decrease sharp injuries. We anticipate an associated decrease in needlestick injuries with the use of the device.'”
Other observations by the authors included the potential for OR efficiency gains, “In addition to the device’s impact on needlestick risk, it may also have the potential to improve efficiency by introducing parallel processing in which two or more separate processes are conducted simultaneously rather than in series.” While the surgeon is closing independently, managing their own sutures, the circulator and scrub-tech can focus on performing their final count and breaking down the room, eliminating conflict between these two equally important activities.
The study concluded that Operative Armour effectively functions as an ESIP device by decreasing intraoperative needle passing and handling. With over 88,000 operating room needlestick injuries in the US each year, Operative Armour provides a new option to help keep Surgeons, Nurses, and other medical staff safer in the operating room.