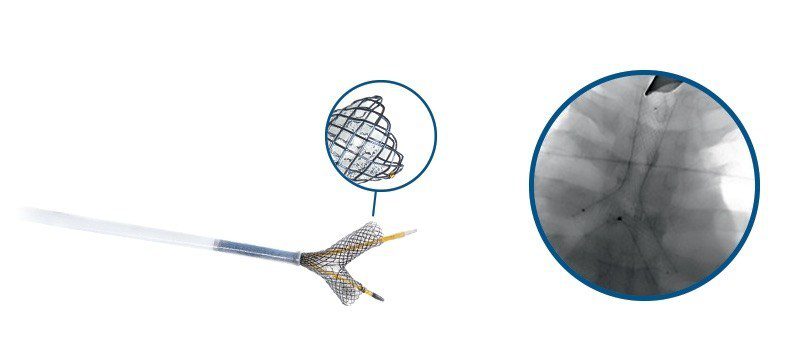
The self-expanding y-shaped tracheal stent system is designed to be a flexible, compliant device to aid in the treatment of malignant neoplasms at the tracheobronchial carina. Micro-Tech Endoscopy announced the news today.
- Low profile delivery system for ventilation and visualization of the stent during placement
- Silicone covering to restrict tumor ingrowth
- Repositioning sutures for adjustment or removal of the stent
The flexible, compliant Y-shaped Tracheal Stent System aids in the treatment of malignant neoplasms at the tracheobronchial carina. The device is effective in providing relief for patients with malignant tracheobronchial strictures due to its nitinol self-expanding design and ease of deployment.
“I use the Micro-Tech y-shaped Tracheal Stent System because it is easy to deliver across carina lesions,” said Chakravarthy Reddy, M.D. at the University of Utah Health Sciences Center. “Its innovative design allows for visualization during placement and does not require suspending ventilation during positioning. The stent walls also provide increased airway diameter when compared to silicone stents.”
Since 2000, Micro-Tech Endoscopy has been focused on creating top-quality products for endoscopic diagnosis, and therapeutic medical devices that allow physicians to provide the highest level of care. By partnering with doctors dedicated to innovation, Micro-Tech is committed to bringing better devices to market, with unparalleled speed, at an economical price, and without the burden of contracts. Micro-Tech does not compromise on quality and does not believe customers should either.
Micro-Tech Endoscopy has operations in America, Asia, and Europe and leverages this global reach to rapidly commercialize and refine the products it brings to its clinician partners. Micro-Tech’s team has a wealth of experience in the field and in-depth understanding of both product and use cases.
With the health care industry transforming rapidly, Micro-Tech Endoscopy is dedicated to setting the pace as a disruptor. Micro-Tech is more than a medical technology company, it is building a community of healthcare innovators and making health care more value-driven.