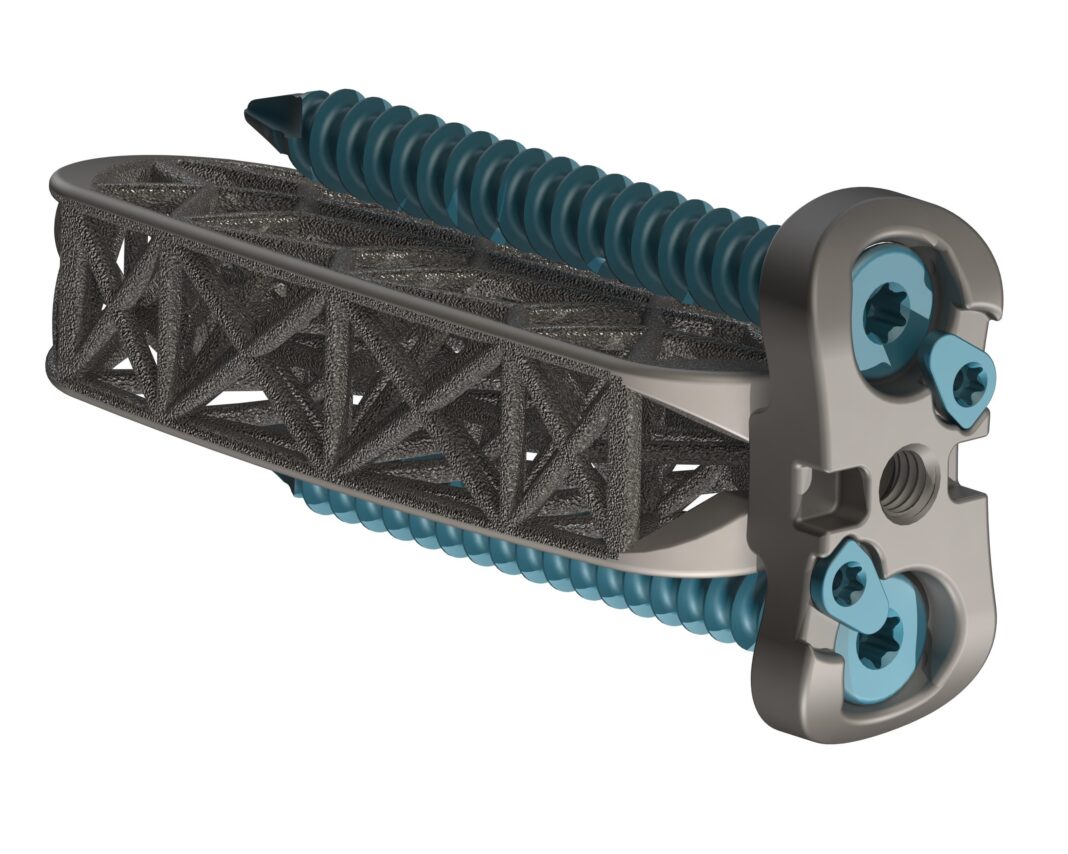
4WEB Medical, an orthopedic device company focused on developing innovative implants with an Advanced Structural Design utilizing its proprietary Truss Implant Technology™, announced today the company’s launch of a comprehensive array of hyperlordotic lateral implants.
4WEB Medical advises that the first procedure was performed by Brad Prybis, MD, a surgeon at Tanner Medical Center in Carollton, GA.
“The application of 4WEB’s Truss Implant Technology within the lateral spine space has positively impacted my practice over the years,” said Dr. Prybis. “The new hyperlordotic implant offering, combined with an already comprehensive lateral portfolio, expands my treatment options for patients who need more extensive sagittal balance correction.”
The newest addition to the Lateral Spine Truss System™ (LSTS) will include implants with 18°, 24°, and 30° lordotic angles to be used for complex spine correction in anterior longitudinal release procedures. These implants, combined with 4WEB’s Lumbar Spine Plating Solution, allow for more cost-effective treatment options and reduce the need for larger, more invasive posterior corrective procedures. The company’s lateral access portfolio also includes procedural-based solutions developed for Direct Lateral, Anterior-to-Psoas (ATP), and Prone Lateral approaches to the lumbar spine.
“The launch of the hyperlordotic lateral implants is the latest in a string of high impact product launches that have fueled record growth for the company,” said Jim Bruty, Sr. Vice President of Sales and Marketing. Revenue from the Stand-Alone Anterior Lumbar Spine Truss System™ (ASTS-SA), Stand-Alone Cervical Spine Truss System™ (CSTS-SA), and Lumbar Spine Plating Solution (LSTS-PS) were primary drivers of core spine revenue growth in 2021. “Largely attributed to these new product launches, 4WEB finished 2021 with nearly 30% year-over-year growth.”
Beyond the Hyperlordotic LSTS launch, 4WEB has plans for three additional significant product launches in 2022 that will further sustain the company’s rapid growth trajectory.