Aerin Medical Inc., a company dedicated to providing Ear, Nose and Throat (ENT) physicians with non-invasive solutions for the treatment of chronic nasal conditions, announced that the American Medical Association (AMA) Current Procedural Terminology (CPT®) Editorial Panel accepted the application for a new Category I CPT code to report endoscopic destruction of the posterior nasal nerve using radiofrequency (RF) ablation.
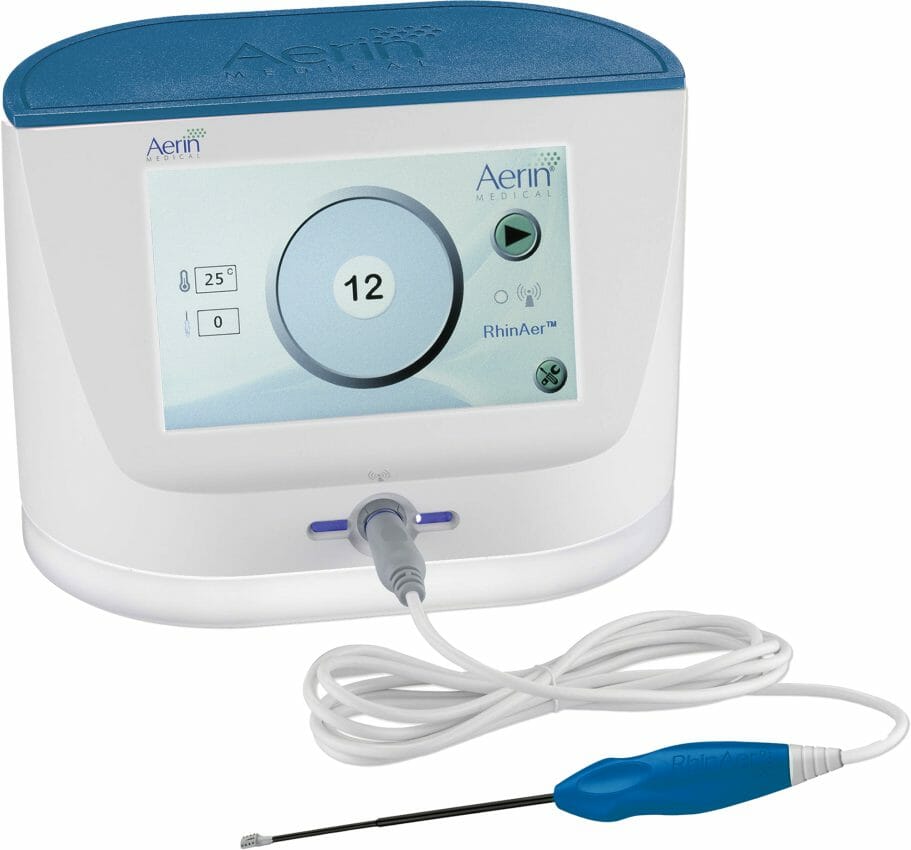
This information was released by the AMA in the Summary of Panel Actions from the September CPT Editorial Panel meeting. The new code will become effective on January 1, 2024, and describes the procedure performed by ENT physicians when treating their patients with Aerin Medical’s RhinAer®. The RhinAer Stylus precisely applies RF energy to overactive nerves in the back of the nose, reducing chronic rhinitis symptoms.
“A new CPT code is an important milestone for ENT physicians and their patients, enabling appropriate valuation and improving access to this transformative solution that can be readily performed in the physician office setting,” said Matt Brokaw, CEO of Aerin Medical. “We appreciate the otolaryngology society leadership and physician investigator commitment to generating robust clinical evidence for the RF approach, including the longest-term published randomized controlled trial data available.”
The company recently announced the launch of a next-generation RhinAer Stylus designed to provide physicians with improved visualization, as well as easier access and tissue apposition, especially in patients with narrow nasal airways.