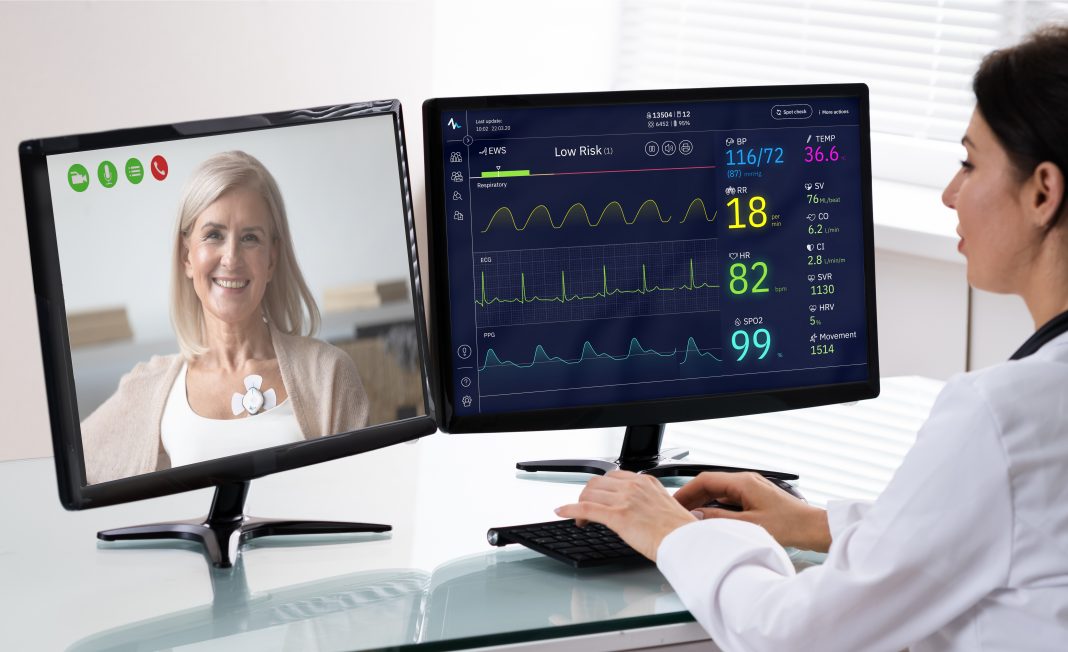
Health AI hospital at home patient monitoring kit has been launched, reports Biobeat.
The AI Hospital at home patient monitoring kit solution allows healthcare providers to remotely monitor patients’ vital signs and receive updates and deterioration alerts, helping to alleviate hospital overload, reduce costs and improve patient care.
Based on the same hospital-grade wearable monitoring sensor technology used in hospitals and care facilities around the world, Biobeat’s home monitoring kit includes chest-monitors, compatible adhesive units and a user-friendly manual. Patients will utilize an accompanying intuitive mobile app (compatible with IOS and Android devices) to share the data with providers and view their own health status. Moreover, the patient data is analyzed by Biobeat’s AI-powered cloud-based patient management system, giving health care staff a continuous view of patient health and predictive patient deterioration alerts.
“Our new hospital-level home-based patient monitoring kit is essential in today’s socially distant pandemic climate,” said Arik Ben Ishay, CEO of Biobeat. “As hospitals continue to grapple with over flooded COVID wards and increasing rates of infection, it is crucial to empower health staff with trusted clinical-grade wearable AI-powered patient monitoring tools that will allow them to provide hospital-level care from a distance. In this way, providers can better manage patient influx, reduce facility expenses and most importantly, improve patient outcomes. We expect to see hospital-level home-based remote patient monitoring solutions become the standard of patient care in 2021 and beyond, strengthening the Hospital at Home concept.”
Biobeat’s FDA-Cleared and CE Marked chest-monitors utilize proprietary non-invasive reflective photoplethysmography monitoring technology to automatically and continuously track multiple vital signs and health parameters. The wireless solutions connect to a cloud-based patient management system to provide medical staff with real-time data and alerts, enabling early identification of clinical deterioration. The management platform includes an integrated automated, customizable early warning score system that incorporates advanced health-AI-based algorithms that analyze aggregated patient data to identify deterioration more accurately and provide predictive analytics. This platform could help patients, their family members, and healthcare providers in multiple settings, allowing to optimize care of patients in the post-discharge and outpatient settings, as well as hospital-level home care of oncology patients, COVID-19 patients, and more.
The new home-based remote patient monitoring system is now available to healthcare providers across the globe.