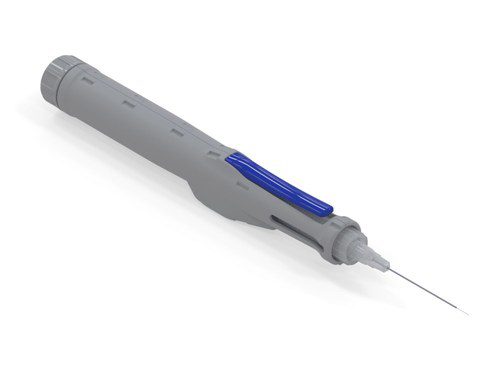
Altaviz, a designer, developer and manufacturer of medical devices and drug delivery platforms, today announced the availability of the groundbreaking, handheld MVI Platform (MVI) for private-label applications.
MVI, or Microvolume Injector, is the world’s first delivery platform ideally suited for self-contained, flow-controlled delivery of gene therapies, stem cell therapies, dermal fillers, and any high viscosity fluid that requires precision dose delivery and control.
The feature rich MVI delivers exacting precision, superior reliability and consistent, controlled dosing through a simple to use, self-contained handheld device. As a platform, MVI is highly versatile and can be adapted to a wide range of applications and therapies previously outside the scope of a handheld form factor.
The patented MVI Platform was originally designed and built to the requirements and delivery standards for the highly demanding sub-retinal injection of gene therapies. Today, MVI is a flexible platform which can be adapted to deliver in the operating room and in out-patient procedures. Altaviz is seeking partners to commercialize the platform for multiple applications and therapies.
“Subretinal delivery of extremely high value gene therapies is one of the most promising and demanding surgical procedures done today,” says Jack Auld, CEO at Altaviz. “The problem is that prior to MVI, there were no methods or devices to enable this critical treatment. But we’ve provided a platform that could solve that. We took a full-system approach to optimize every detail of the procedure…and, we’ve now optimized it as a platform that can be adapted to other uses as well.”
The MVI Platform was conceptualized, designed and developed through the Company’s extensive innovation methodology. The result of this integrated and holistic approach to solution engineering has resulted in a platform which could solve a known and long-standing industry gap while providing an elegant, easy to use, cost effective solution packed with novel features and capabilities.
Handheld Simplicity
This self-powered device comes in a small, easy to operate form factor and can be used with single handed operation.
High Viscosity Therapies
The unique high-power drive system delivers the full range of viscosities from equivalent gene therapies to the highest viscosity dermal fillers through 41ga equivalent cannulas.
Flow Control
Built to provide exceptional and flexible Flow Control, MVI provides a consistent experience across varying downstream resistances. If used as a subretinal injector, this could translate to the unsurpassed bleb initiation and consistent formation surgeons have been looking for.
Dose Precision
To ensure drug and therapy dosing accuracy, the MVI has fully integrated visual and audio dose feedback cues to increase the surgeon’s ability to hit the dose target.
Dose Control
The integrated dose tracking system is designed to continuously measure dosing with sub-microliter precision. MVI automatically records injection metrics both on-board and through external endpoints (tablet, laptop or personal computer).
Consistent Reliability
Multiple feature innovations combine to enable superior delivery reliability and consistency. The gas-powered hydraulic drive mechanism produces repeatable and precise delivery rates. Meanwhile, the sterile, single use device form-factor ensures device to device integrity and consistency.
Bluetooth Communications
The MVI Platform has been built with embedded wireless connectivity through Bluetooth. Simply connect MVI to a supported endpoint (tablet, laptop or personal computer) for real-time logging and application control.
“We are thrilled to bring the MVI Platform to fruition,” says Auld. “Patients, physicians and pharmaceutical manufacturers are anxiously awaiting a new way to deliver the industry’s innovative new high viscosity drugs and therapies. The MVI platform could improve healthcare and enable a new generation of treatments.”