January 27, 2021
Amplifi Vein Dilation System, successfully completed first human use has been reported by Artio Medical, Inc. The first clinical procedure was performed by Adrian Ebner, MD, the Head of the Cardiovascular Department at Sanatorio Italiano Hospital in Asuncion, Paraguay.
An arteriovenous fistula (AVF) is the preferred type of vascular access for most end-stage renal disease patients requiring hemodialysis, and clinical research has shown that AVFs last longer and have fewer complications than other forms of access.1 Currently, about 35% of U.S. hemodialysis patients are ineligible to receive an AVF, predominantly due to small vein diameters.2 Of those patients who are eligible, more than 50% of AVFs fail to mature without additional procedures, and many of these sites are abandoned prior to routine use.2 Studies suggest baseline vein diameter may play an important role in eligibility for AVF surgery, and in achieving AVF maturation and use.3
“Vascular access is a constant challenge for end-stage renal disease patients who depend on hemodialysis treatment. Most patients are plagued with interrupted or delayed care due to repeated access site failures and require additional procedures and surgeries to maintain vascular access,” commented Dr. Ebner. “Many also frequently experience access site complications, often resulting in hospitalization and the need for additional care. I am pleased to be a part of the first human use for the Amplifi system. This is the first technology that seeks to address these challenges by proactively preparing patient’s veins for AVF creation.”
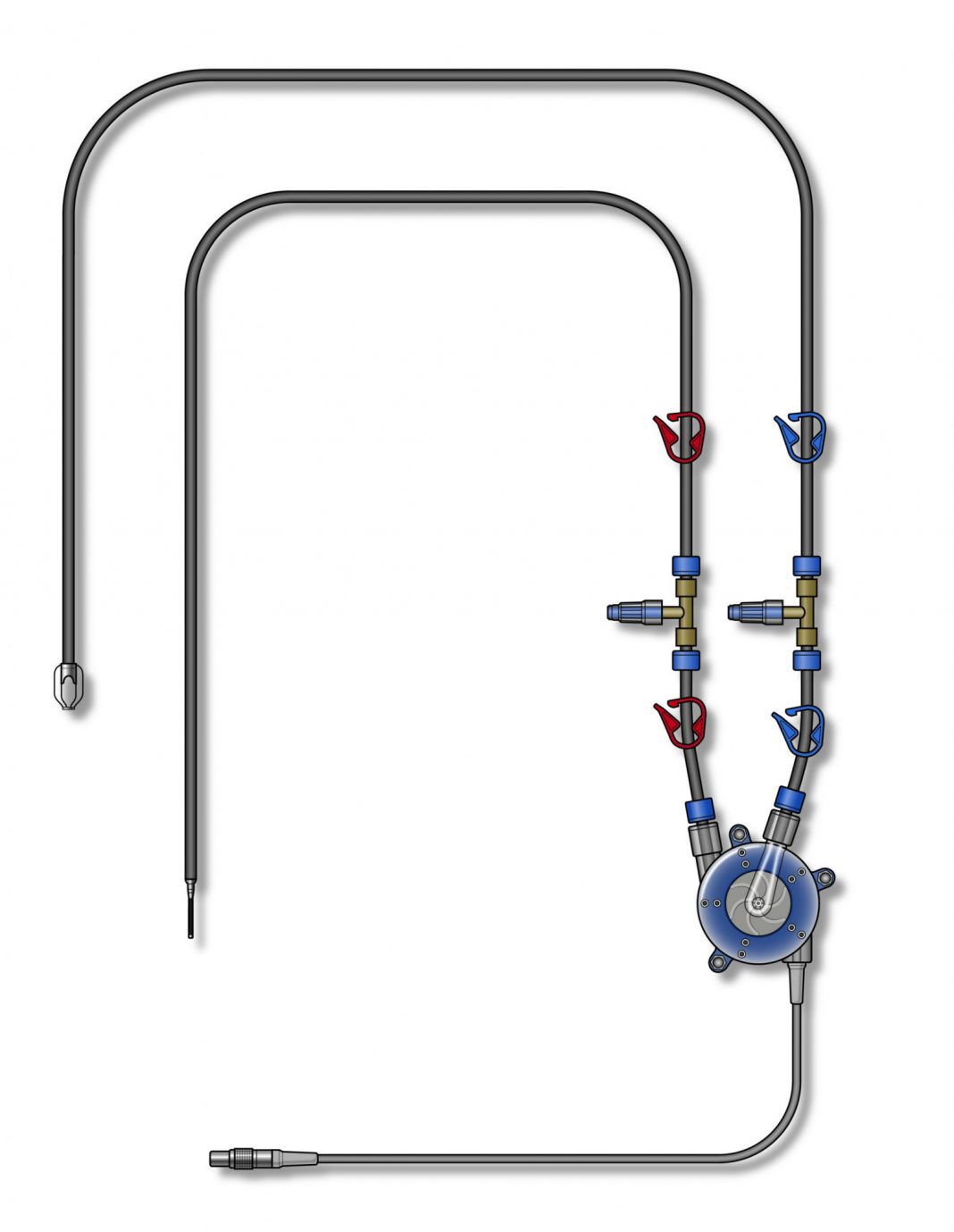
Artio Medical’s Amplifi Vein Dilation System is designed to stimulate arm vein enlargement in hemodialysis patients using rapid, non-pulsatile blood flow. The innovative system is designed for percutaneous placement and includes a wearable, external blood pump, inflow and outflow catheters, and a controller. The Amplifi system is used for 7 to 10 days and removed completely during AVF creation.
“I believe this device has the potential to change the standard of care for hemodialysis patients, allowing more patients to be eligible for AVF surgery and reducing the risk of AVF failure and abandonment,” continued Dr. Ebner. “The degree of vein dilation we observed during the treatment period for the first patient was remarkable, and the AVF made with the treated vein matured very rapidly.”
“Artio Medical is committed to developing novel devices that have the potential to provide better patient outcomes,” stated F. Nicholas Franano, MD, President and CEO of Artio Medical. “Many thanks to Dr. Ebner and the entire clinical team at Sanatorio Italiano for helping Artio reach this important milestone. We look forward to sharing the results of our first clinical experience with the Amplifi system.”
Artio Medical acquired the first-of-its-kind vein dilation technology through the acquisition of Flow Forward Medical, Inc. in June 2020. The Amplifi Vein Dilation System aims to address common issues related to vascular access site creation and maintenance for the 2.3 million patients worldwide with end-stage renal disease who require hemodialysis.2 Artio expects to complete the first-in-human clinical study in the first half of 2021.