September 16, 2020
AOTI Inc. announced today an eagerly anticipated expansion of its unique multi-modality Topical Wound Oxygen (TWO2) therapy product family.
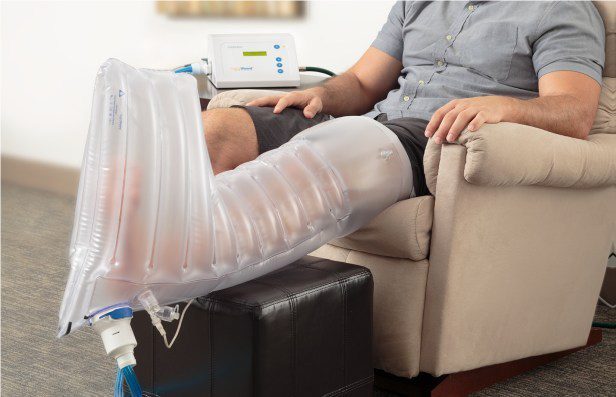
AOTI notes the addition of a new larger extremity-chamber to the company’s peripheral vascular disease (PVD) focused product line, allows for the application of TWO2 treatments for many more patients suffering from costly nonhealing chronic wounds. Especially addressing the needs of those with larger limbs, or with venous leg ulcers (VLU) being treated by conventional compression dressings (CCD) or UNNA boots, or those with diabetic foot ulcers (DFU) being offloaded using a total contact cast (TCC).
Each year more than 8 million people in the USA suffer from chronic wounds that are commonly associated with such endemic comorbid health conditions as Diabetes, Obesity and PVD. TWO2 therapy has been shown to be particularly helpful in treating edematous wounds, by providing cyclical-pressure oxygen to help reduce edema via noncontact compression. TWO2 treatments are applied at home without the need to remove gas permeable dressings, with oxygen diffusing easily through CCD, UNNA boots and TCC adjunctive treatments. TWO2 has been further shown to reduce inflammation and help combat infection, thereby stimulating angiogenesis, while promoting better quality collagen synthesis, resulting in more robust and durable wound healing for up to 36 months. (1, 2)
“Prescribers had requested a larger version of our extremity-chamber, so they could treat the nonhealing wounds of patients that simply could not fit into the medium-sized chamber, due to either the size of their limbs or because they were also being treated with other adjunctive therapies, such as total contact casting and conventional compression dressings. We heard this plea and responded quickly to expand our product range. We are proud to be able to now provide the proven durable-healing outcomes of TWO2 to many more chronic wound patients in combination with their mainstay treatments,” stated Dr. Mike Griffiths, CEO and Medical Director of AOTI Inc.
Once ordered by the prescriber and set-up by our dedicated care team, TWO2 therapy is easily applied daily by the patient at home, without the need for clinic visits related to its utilization. AOTI also provides the prescriber with complimentary “eyes on the wound” Telehealth feedback, allowing for both healing and wound protective care to be delivered safely for patients at home. Something that has been shown to be vitally important during the COVID-19 pandemic.
- Diabetes Care 2020;43:616–624 | https://doi.org/10.2337/dc19-0476
- Vascular and Endovascular Surgery; 47(1),30-37 (3) I https://doi.org/10.1177%2F1538574412467684