TekniPlex Healthcare, which utilizes advanced materials science expertise to help deliver better patient outcomes, will showcase its recently expanded range of paratubing solutions at COMPAMED Hall 8b, Stand N02, November 14-17 in Düsseldorf, Germany.
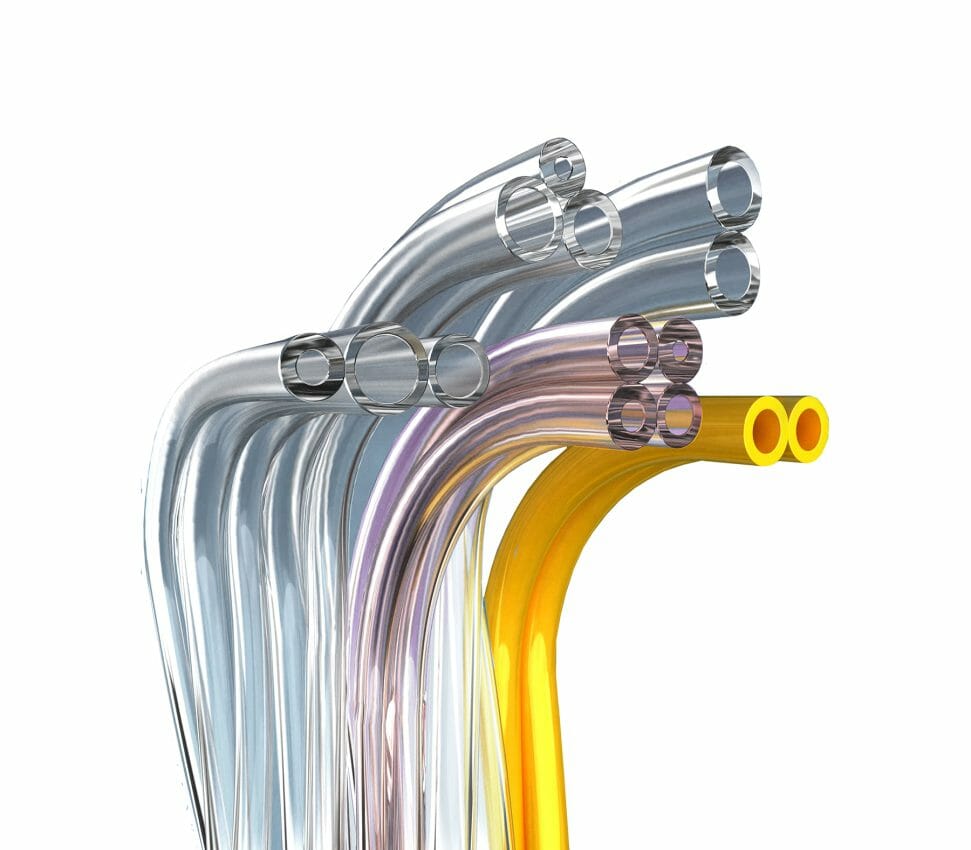
Among other differentiating benefits, TekniPlex Healthcare’s paratubing solutions can be produced in up to eight tube configurations, including custom-colored, textured or striped tube formations to distinguish between flow paths.
Paratubing consists of multiple, single lumen tubes bonded together for operations such as arterial drills or biopsies, as well as with medical devices utilized for wound management, laparoscopic, neurovascular and ophthalmic procedures. Paratubing makes it easier for clinicians by eliminating the need for clips and ties commonly used to organize multiple tubes, allowing each individual tube to be peeled apart and affixed to its relevant medical device or equipment for suction, irrigation, drug delivery or lighting.
TekniPlex Healthcare’s validated manufacturing processes yield paratubes with uniform, repeatable and consistent bonding strength between 0.22 and 1.5 pounds, protecting dimensional stability and ensuring each individual tubing line’s integrity. Maintaining consistent bonding strength prevents individual tubes from separating prematurely and being rejected by healthcare providers, and also eliminates the need for excessive force to peel the tubes apart, which can damage or distort them to the detriment of patients.
“Medical device manufacturers need to consider the implications of tubing design, construction and assembly on device performance, as patient outcomes can be affected,” said Chris Qualters, CEO of TekniPlex Healthcare. “Our validated manufacturing processes and rigorous inline testing ensures that paratubing lines consistently meet stringent, diverse device specifications. The result is improved accuracy, efficiency and safety.”
Manufactured with tight inner and outer diameter tolerances, TekniPlex Healthcare’s paratubing solutions are available in standard PVC and non-phthalate PVC formulations engineered to accommodate unique device specifications. Customized solutions can include tubing enhancements to assure correct and secure line connection with device ports, fittings and luers. Custom validation services also are available.
Also at COMPAMED, TekniPlex Healthcare will showcase its new lower-weight reinforced coated papers for medical device protection. The company’s proprietary coating formulations and application technology result in rolls that can meet demanding performance and sterilization requirements, even when slimmed down.
The new reinforced coated papers are suitable for packaging a variety of common healthcare items including syringes; tubing such as IVs, catheters and airway filters; gauzes, sponges and bandages; and devices sterilized by EtO and radiation. TekniPlex Healthcare also will display samples of its TekniMDo® PX, a high-performance thermoformable co-polyester film serving as an alternative to PETG for medical device packaging applications.