ATF Medical has introduced its Pressure Injury Prevention and Intervention (PIPI) program to make sure that everyone involved in a workers’ compensation case has the information needed to prevent or properly treat pressure injuries, also known as wounds.
“There is a large body of information from credible nursing, rehabilitation, and equipment manufacturers about pressure injuries, but it is not all in one place, and not customized to the individual and circulated to all the providers who need it,” said ATF Medical’s Director of Rehab Technology Edwina Murphy, OTR, ATP.
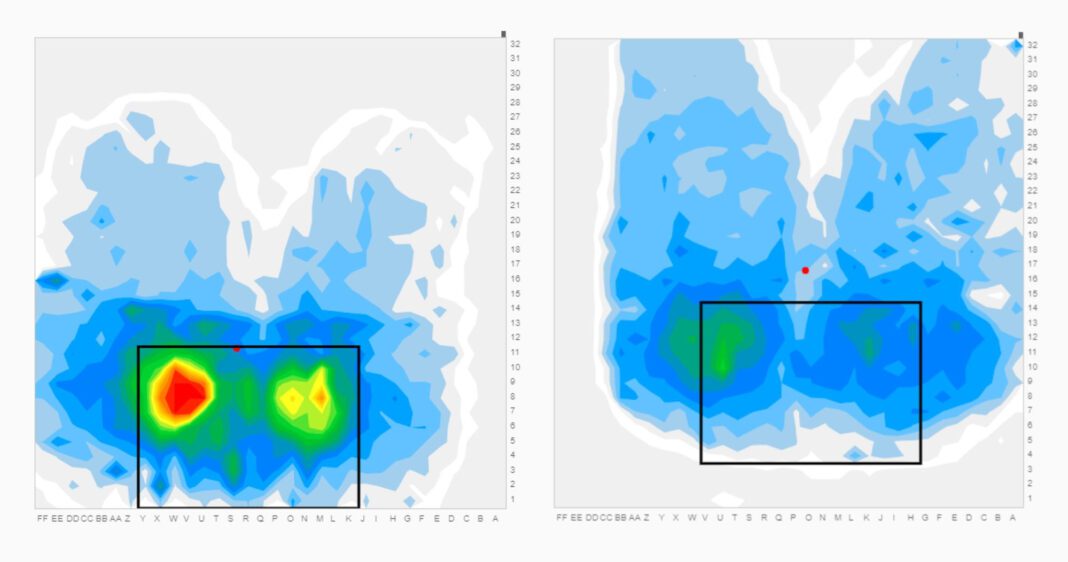
The PIPI program reviews clients’ claims to identify injured workers at risk of developing pressure injuries and recommends ways to avoid them. When wounds already exist, ATF Medical’s professionals evaluate the injured worker’s condition and environment and uses pressure mapping systems for chairs and mattresses to detect pressure points.
“Pressure mapping helps identify a seat or mattress issue, often something that can be easily and inexpensively adjusted,” Murphy explained. “It’s an eye-opening educational tool. When injured people can see how relieving pressure affects the colors on the map, they’re more apt to comply with relief techniques and other self-management guidance.”
ATF Medical designs a customized pressure management program for the injured worker, synthesizing relevant treatment protocols, durable medical equipment data, and educational materials, and shares it with the injured worker, providers, and the claim representative. The report recommends solutions, such as pressure relief schedules to increase circulation or obtaining power-assisted tilt for a person who cannot move him or herself.
“Our program is designed to avoid and reduce costs through proper communication, coordination and accountability,” Murphy said. For more information on the ATF Medical Pressure Injury and Prevention Program, please contact Murphy at emurphy@atfmedical.com.