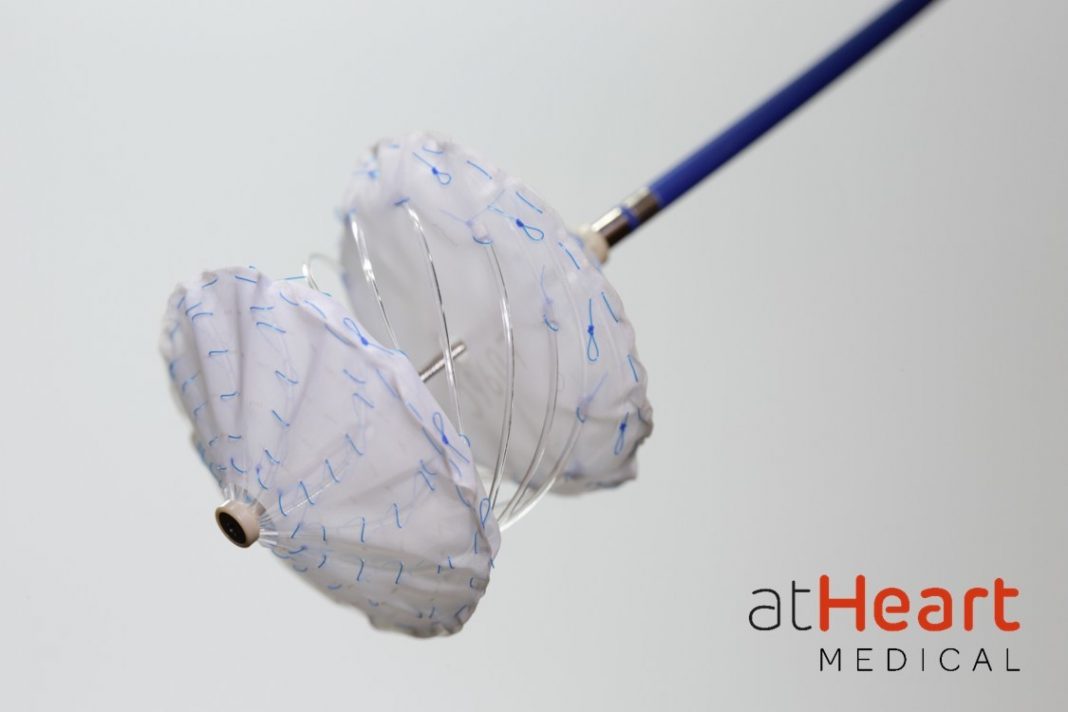
atHeart Medical, a medical device company dedicated to establishing the new standard of care for closure of atrial septal defects (ASD), today announced the appointment of Andrew Cleeland to the company’s Board of Directors.
With over thirty years of experience, including specific expertise bringing game-changing technologies to market, Mr. Cleeland joins as the company builds momentum for the ASCENT-ASD U.S. investigational device exemption (IDE) trial studying the safety and efficacy of its reSept™ ASD Occluder technology.
“I am excited to join atHeart’s board and work with the company to evolve septal closure. The company’s next-generation, metal-free, bioresorbable frame design is a novel approach in the structural heart space,” said Mr. Cleeland. “Unlike current competitive metallic frame occluders, the reSept occluder resorbs over time to restore a more natural septum and leaves minimal metal behind, a significant benefit for patients who may require future transseptal interventions.”
Mr. Cleeland is currently the CEO of Fogarty Innovation, a leading nonprofit focused on visionary approaches that address unmet needs in an evolving medtech landscape. Prior to joining Fogarty, Mr. Cleeland served as vice president and general manager at Medtronic following the acquisition of Twelve Inc., which he led as CEO. Previously, he was the president and CEO of Ardian, the pioneer of renal denervation for hypertension acquired by Medtronic in 2011. He currently serves on the board of three venture-funded companies, Saluda Medical, Zenflow, Inc. (chairman) and MMI S.p.A. (chairman). Mr. Cleeland also holds advisory positions at two top-tier venture capital firms, Longitude Capital and Arboretum Ventures. At a global industry level, he has been invited to serve on multiple initiatives including the UCSF-Stanford Pediatric Device Consortium, the Medical Device Innovation Consortium, and the Singapore government’s Biomedical Research Council (BMRC).
“We are pleased to welcome Andrew to the atHeart board. He is a proven medtech leader with a strong passion for championing novel healthcare solutions,” commented Laurent Grandidier, CEO of atHeart Medical. “His wealth of knowledge and business expertise will be critical in guiding our strategic plan as we accelerate the IDE trial and prepare for future milestones.”