In mid-2021 Basil Systems launched its innovative SaaS platform dedicated to simplifying navigation of healthcare data. Their platform revolutionized how Med-Tech companies conduct regulatory and market research with the goal of getting safe and innovative products quickly to market.
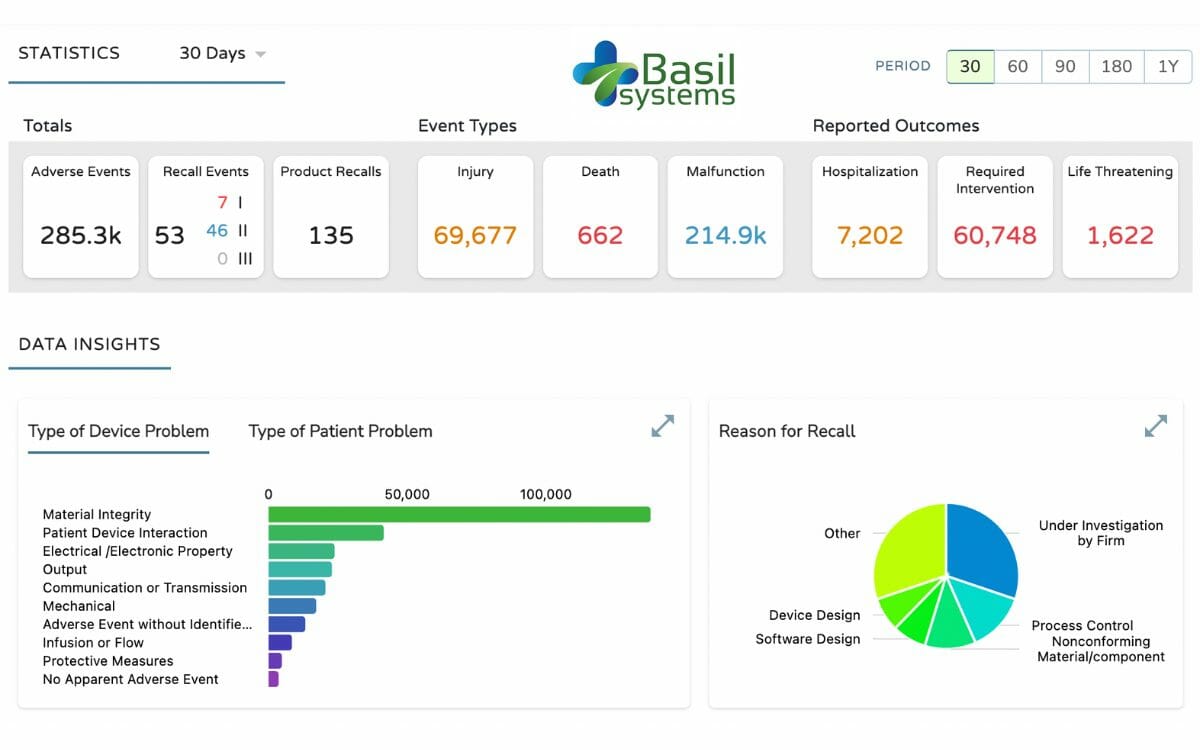
Now, Basil Systems has commercially launched its Post Market Intelligence platform. Another SaaS product, The Post Market Intelligence platform is a first of its kind, easy-to-use research toolset for post market quality, safety, and compliance data such as recalls, adverse events, warning letters, inspections, and more.
With the Post Market Intelligence platform companies can expect to dramatically decrease their time spent on quality and surveillance research analytics, while simultaneously increasing the amount of truly valuable insights generated.
The Basil Systems Post Market Intelligence enables creation of personalized “datasets” – customized views into industry-wide quality and product data, tailored to your specific market. A dataset consists of any combination of companies, brands, or categories desired, to be further analyzed independently or with comparison to competitor data.
One of the innovative features this enables is the personalized alerts based on the custom datasets created. These alerts offer immediate notifications directly to your inbox when a recall, adverse event spike, or warning letter is issued in the topic of interest the user defined.
A huge challenge today is the high cost and effort demanded by the recent European MDR/IVDR regulations regarding mandatory resubmissions to keep products on the EU market. Within the Post Market Intelligence platform, Basil Systems offers an essential new capability: CER/PSUR data table creation. Users can create CER/PSUR tables quickly, easily, and fully, comparing post-market quality data across companies and brands instantly. These CER/PSUR tables that previously took weeks (or tens of thousands of consultant dollars) to create, now can be created, adjusted, and refined in just minutes.
The Post Market Intelligence platform is the beginning of a new era in quality data surveillance. With the ability to be highly confident in data analytics that are also easy and fast to navigate, the healthcare industry can efficiently survey products on the market for further improvements, quickly find and solve device issues, and ensure quick action dedicated to user safety.
Basil Systems plans to continue bringing new and revolutionary solutions related to data transparency, accessibility, and analytics in the healthcare industry to create a better experience for both companies and patients.
Authors note:
Basil Systems – Founded in 2019, Basil’s platform leverages machine-learning across product, post-market and regulatory data to provide analytics and insights that guide strategic product development decisions, improve product performance and supply chain management, mitigate recall risks, and significantly reduce regulatory approval times.