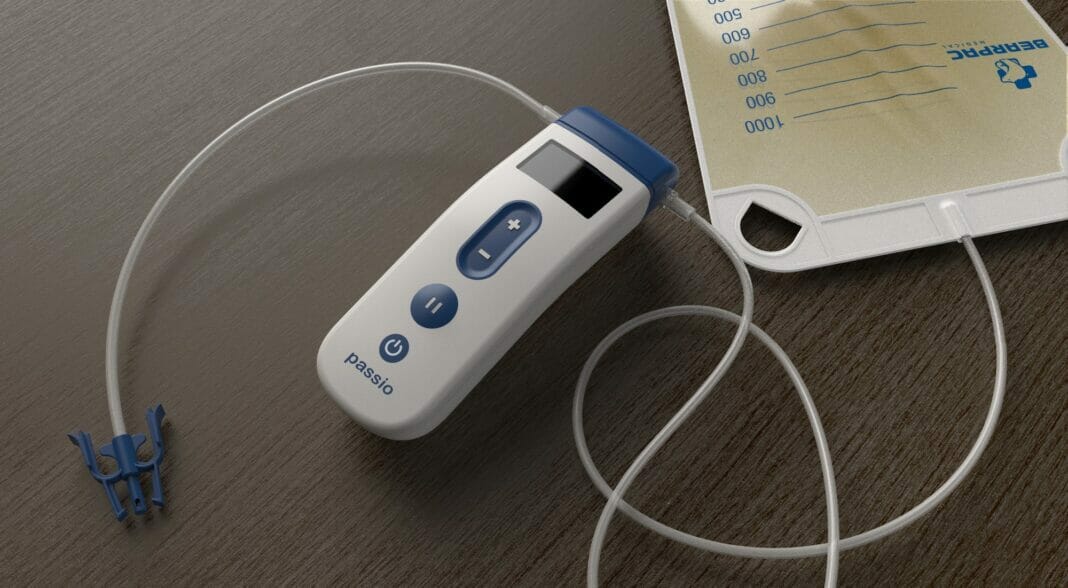
Bearpac Medical, LLC announced that it has entered into an exclusive distribution agreement with APR Medtech Ltd (Thame, Oxon, UK) to sell the Passio Pump Drainage System, indicated for the intermittent drainage of recurrent and symptomatic pleural effusions and malignant ascites.
The Passio Pump Drainage System consists of the Passio Catheter, a Handheld Control Unit (pump) and a Disposable Collection Kit, which includes a redressing kit, for drainage of recurrent and symptomatic pleural effusions and malignant ascites. The Passio pump is attached to an implanted Passio catheter using the disposable collection kit and is activated to begin the evacuation of fluid into the collection bag. The Passio catheter is exclusively designed for use with the Passio collection system. Passio provides flow control throughout the therapy at lower vacuum pressures than competitive devices on the market today.
“This agreement with APR Medtech presents a significant market opportunity for our Passio Pump Drainage System, now with an expanded CE certification to include both pleural and peritoneal indications” said Jay Zimmerman, President of Bearpac Medical.
“The Passio Pump Drainage System is an innovative, next generation product and we are delighted to be partnering with Bearpac Medical to bring this technology to the NHS and UK private healthcare sector ” said Michael Pichel, Technical Director of APR Medtech Ltd.