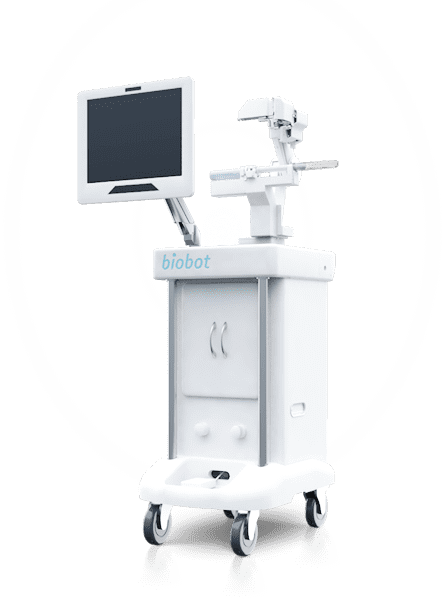
Biobot Surgical is excited to announce U.S. FDA 510(k) clearance of the iSR’obot Mona Lisa 2.0. The new robotic assisted transperineal needle-guidance system allows urologists to plan and position a single needle or multiple needles during image-guided diagnostic and interventional prostate procedures.
Biobot Surgical notes the iSR’obot Mona Lisa 2.0 is the latest addition to Biobot Surgical’s iSR’obot Mona Lisa product portfolio. “The Mona Lisa system was conceptualized and engineered to position biopsy or treatment needles transperineally. The advantages of transperineal procedures are lower infection rates and better coverage of the anterior zone compared to transrectal procedures. In addition, most ablation procedures are also carried out transperineally. The Mona Lisa system simplifies needle positioning by providing robotic assisted guidance for the insertion.” said Sim Kok Hwee, CEO of Biobot Surgical.
The iSR’obot Mona Lisa 2.0 automatically supports needle positioning during transperineal prostate biopsy and prostate cancer ablation based on a customized needle plan.
The new features include:
- A detachable needle guide that holds a biopsy needle or multiple ablation needles
- Visualization and planning of the needle locations for a prostate cancer ablation procedure
- Ability to re-adjust a target location when the needle deflects
The second-generation iSR’obot Mona Lisa system retains the MR-ultrasound image-fusion prostate biopsy technology. The robotic system’s proprietary dual-cone needle trajectory technology enables the extraction of multiple biopsy cores through the same needle entry points. The first-generation iSR’obot Mona Lisa is marketed in Europe, Australia, and Asia. Clinical studies have demonstrated that iSR’obot Mona Lisa has a clinically significant prostate cancer detection rate of 81 percent higher than cognitive fusion biopsy. Clinical data also shows that the transperineal, dual-cone needle trajectory technology minimizes infection complications[1-4].
Biobot Surgical Pte. Ltd is headquartered in Singapore and focuses on medical robotics solutions. The company is expanding its core technology in precision needle positioning to address comprehensive prostate cancer care to improve patient prostate cancer outcomes.