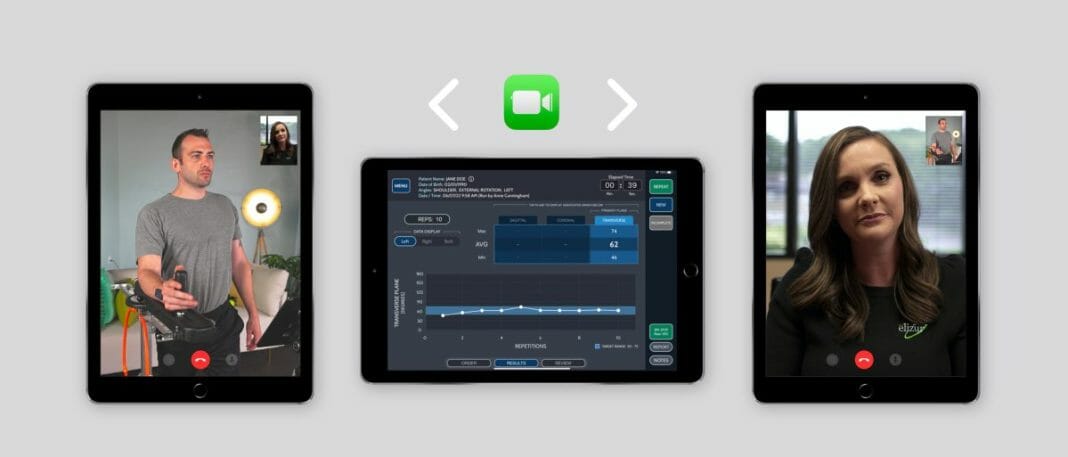
BioMech Health, a division of BioMech Holdings, LLC, and ēlizur, a leading musculoskeletal solutions provider, announced today a groundbreaking agreement to integrate BioMech Lab™ Into the Shoulder Strengthening and Stabilization System (SSS).
The national partnership brings together two industry leading mechanical and digital solutions to create an in-home product capable of quantifying rehabilitative outcomes while increasing patient adherence to and engagement with physician-directed physical therapy protocols.
“Coupling the SSS advanced mechanical technology and BioMech Lab’s cutting-edge motion analytics is the perfect union to deliver enhanced therapeutic decision support for improved patient compliance with prescribed physical therapy protocols. It also provides the quantifiable outcomes needed to confirm the reliability of the SSS device,” said BioMech Founder Frank Fornari, Ph.D.
BioMech Lab™ delivers to clinicians the real-time functional motion metrics needed to rapidly evaluate, design, and monitor physical, surgical, pharmaco, and cognitive therapies. Using advanced sensor technology, powerful artificial intelligence (AI) and machine learning (ML) algorithms, and interactive biofeedback, BioMech Lab captures motion data in clinical and real-world settings and delivers precise, accurate, and reproducible assessments and treatment modifications that stratify risk and improve care outcomes.
“SSS has helped more than 4,000 patients accelerate rehabilitation from injury or surgery while reducing the number of required outpatient sessions. Adding BioMech Lab’s powerful analytics and biofeedback capabilities creates an even more robust system that can now deliver the actionable information needed to maximize outcomes while lowering costs,” said ēlizur President Jim Grant.
The SSS is designed for early-stage in-home rehabilitation use in the interim between discharge and outpatient therapy. A complement to outpatient rehabilitation, SSS offers a convenient, cost-effective alternative for patients who are not yet ready for, or do not have easy access to outpatient rehabilitation centers. ēlizur virtual clinical specialists – physical therapists, athletic trainers and orthopedic nurses depending upon the patient’s specific need – support the patient with set up and compliant use of the SSS device based on the physician-prescribed therapeutic protocol.
Says Fornari: “The BioMech Lab-SSS combination leverages automation to transform an integral but subjective assessment process and delivers quantifiable metrics to measure progress and validate effectiveness of therapeutic protocols.”