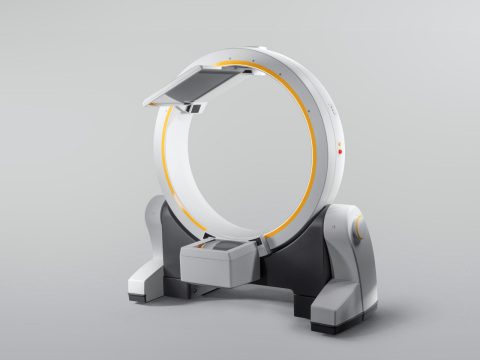
Brainlab notes following on CE mark approvals last summer, the FDA clearance paves the way for Brainlab to now enter the US markets with the Cirq robotic alignment module for spine procedures, and Loop-X, the first fully robotic intraoperative imaging device on the market.
The first-of-its-kind, Loop-X works seamlessly with the full Brainlab digital surgery portfolio or with a customer’s existing surgical setup. Independently moving imaging source and detector panels enable flexible patient positioning and non-isocentric imaging which reduces the amount of radiation exposure and increases the variety of indications which can be treated. This mobile imaging robot can be controlled wirelessly with a touchscreen tablet. Loop-X was developed in close collaboration between Brainlab and partner medPhoton based in Salzburg, Austria, where the first Loop-X was installed. Hospital San Juan de Dios León in Spain recently performed the world’s first navigated spine surgery using Loop-X mobile imaging robot technology.
Following on the success and growing install base for Cirq, a universal platform for robotic tasks, the new Cirq Robotic Alignment module is capable of fine tuning the alignment to a pre-planned trajectory and freeing up surgeons’ hands, enabling them to focus on the patient’s anatomy. Surgeons at Royal London Hospital in the United Kingdom have already used Cirq Robotic Alignment for a range of cases from routine lumbar fusions to complex deformity and cervical fractures.
“We’re expanding and diversifying our digital surgery portfolio with robotics across all indications,” said Sean Clark, President, Brainlab, Inc. “Our customers want to offer their patients advanced technologies close to home. Brainlab technologies are designed to enable greater freedom for clinicians and enhance outcomes for patients.”
The recent availability of these robotic medical devices is bringing a futuristic vision of the modern digital operating room closer to reality supporting surgeons in their day-to-day work and bringing benefits to their patients.