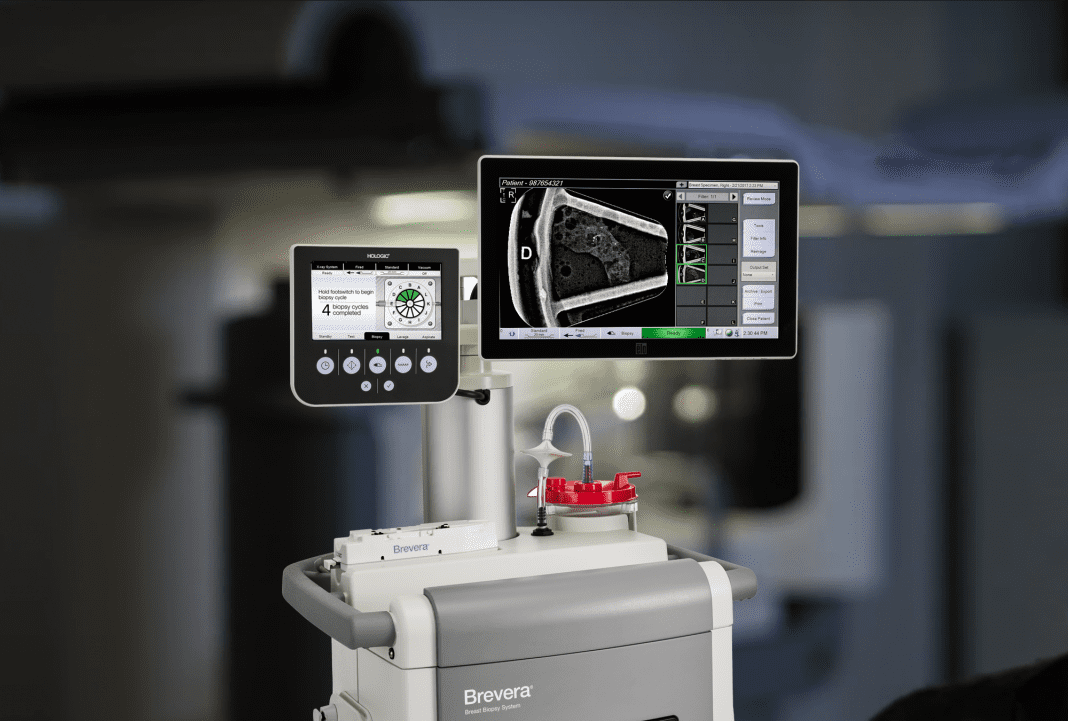
Hologic, Inc. an innovative medical technology company primarily focused on improving women’s health, today announced improvements to the Brevera® Breast Biopsy System with CorLumina® Imaging Technology, the world’s first and only breast biopsy solution to combine vacuum-assisted tissue acquisition, real-time imaging verification and advanced post-biopsy handling in one, integrated system.
Since its launch in 2017, the Brevera system has shifted the way clinicians perform breast biopsies and verify that the targeted tissue samples have been acquired. The system streamlines the entire biopsy process from start to finish – with real-time imaging for verification of sample acquisition and automated post-biopsy specimen handling. These integrated features offer the potential to save facilities an average of 13 minutes per procedure2 to transform the patient experience and enhance workflow. In addition, they allow clinicians to remain by the patient’s side during the entire procedure, eliminating the need to leave the room to image and verify tissue samples, which often leads to lengthy procedure times and anxious patients, as well as interruptions to facility screening schedules.
“As a world leader in breast health, we take great pride in our commitment to constant innovation – informed by clinical research and customer feedback – to continuously expand and improve upon the performance of our products,” said Jennifer Meade, Hologic’s Division President, Breast and Skeletal Health Solutions. “The launch of the Brevera system represented a major transformation in breast biopsy, and we’re confident that the improvements we’ve introduced will further the system’s ability to enhance workflow, increase accuracy and improve the overall patient experience.”
To better meet clinician needs, the Brevera system features a reusable device driver and disposable needles to simplify storage and improve waste management.1 The system also features improvements that allow radiologists and technologists to better separate and verify target samples automatically, then transfer seamlessly to pathology with minimal handling to help maintain core integrity.
The Brevera system is designed for use with Hologic’s Affirm® Prone biopsy guidance system. When paired together, the Brevera system and Affirm® system accelerate the speed and efficiency of prone biopsy procedures to further reduce the patient’s time under compression.[2]
References:
[1] Compared to Eviva® device. Hologic Data on file 2017.
2 Brevera Pulse Survey, Quantitative report. Inspired Insights. April 2019
[2] Results from, “A Prospective, Block Stratified Clinical Trial to Evaluate the Performance and Operation of the Brevera® Breast Biopsy System” (NCT03300206) (US)