November 30, 2020
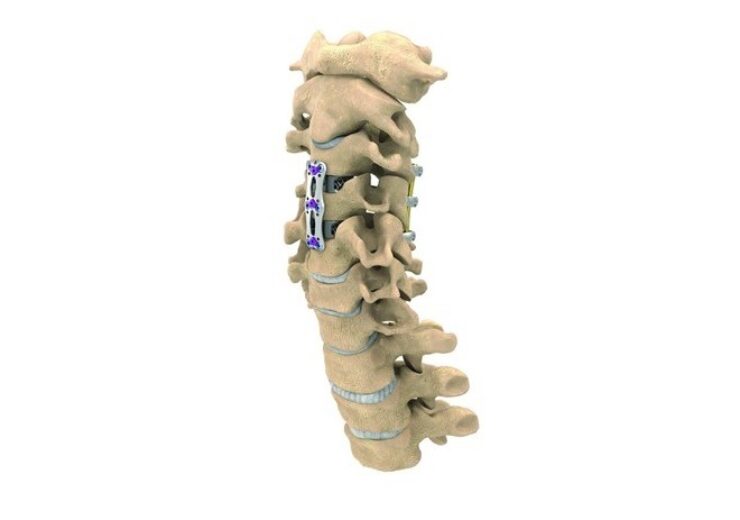
NuVasive has expanded its product line with the introduction of a new C360 cervical spine portfolio. The company has also commercially launched its new anterior cervical plating (ACP) system, which is claimed to feature the thinnest plate on the market at 1.6mm.
NuVasive has designed a new ACP system to minimize common postoperative complications such as dysphagia, malalignment, and adjacent level ossification.
The system helps surgeons to customize treatment as per customer requirements compared to the traditional one-size-fits-all approach.
Features of new anterior cervical plating system
The new ACP system features three plate profiles, including a thin-plate for one and two levels, and offers optimized plate stiffness for each surgical level to support construct stability.
NuVasive’s system offers a range of implant length options to match different patient anatomies and maximize the distance from the adjacent levels.
In addition, the system consists of a range of advanced screw offerings with integrated locking covers to facilitate better screw placement and locking accuracy.
The C360 portfolio also includes the Reline Cervical posterior fixation system, which is expected to be commercialized in 2021.
NuVasive executive vice president Massimo Calafiore said: “As the leader in spine technology innovation, NuVasive saw an opportunity to procedurally integrate technology to support the cervical spine—as it did in the lateral market with X360—to optimize the surgeon experience and advance care in the operating room.
“The launch of C360 is key to our long-term strategy and represents a significant opportunity for growth, as cervical spine procedures comprise an approximately $2.6 billion segment of the global spine market.”
NuVasive offers procedural solutions, including access, implants and fixation systems, biologics, software for surgical planning, and navigation and imaging solutions.
In November, NuVasive expanded its Porous PEEK portfolio with the commercial introduction of a new Cohere extreme lateral interbody fusion (XLIF) system.